Antifungal drugs are a special group of drugs that are used to treat fungal diseases.
Fungal diseases (mycoses) are divided into systemic (affecting internal organs) and superficial (localized) mycoses.
The most common are superficial mycoses - fungal diseases of the skin (dermatomycosis - trichophytosis, microsporia, pityriasis versicolor) and nails (onychomycosis), as well as mycoses caused by fungi of the genus Candida (candidiasis) - vaginal candidiasis (or thrush).
Systemic mycoses are quite rare, mainly in patients with reduced immunity (congenital immunodeficiency, AIDS, after organ and tissue transplantation, during treatment of malignant tumors). Often the source of systemic mycoses in such patients is intravascular catheters contaminated with fungal spores, which are placed during hospital treatment for several days for regular infusions of drugs.
Antifungal drugs are used to treat both systemic and superficial mycoses.
Effective treatment of systemic mycoses, in addition to antifungal drugs, additionally includes the correction of immune disorders, as well as the mandatory elimination of sources of infection (in particular, intravascular catheters).
Indications for use
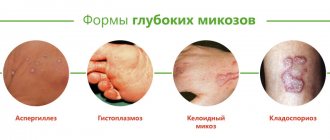
Antifungal drugs are used for diseases caused by pathogenic fungi - mycoses: deep (or systemic) mycoses, in which fungi affect internal organs and the central nervous system - aspergillosis, cryptococcosis, coccidioidomycosis, histoplasmosis, blastomycosis; superficial mycoses (or dermatomycosis) of the skin, scalp, nails - pityriasis versicolor, microsporia, trichophytosis, epidermophytosis, onychomycosis, mycosis of the ear canal.
In addition, antifungal drugs are used for candidiasis of the mucous membranes of the oral cavity, pharynx, esophagus, skin, genital organs - vaginal candidiasis, candidiasis of internal organs.
Nizoral
The main active ingredient of Nizoral is ketoconazole. This drug has a pronounced antifungal effect and is ideal for the treatment of mycosis of toenails. Ketoconazole penetrates deep into the nail and prevents further proliferation of pathogens, destroying their metabolic processes. For optimal therapeutic effect, it is recommended to apply Nizoral to the affected areas twice a day. The indisputable advantage of this remedy for fungus is that it is completely safe for the body. Pregnant and lactating women can be treated with Nizoral.
Nizoral
Janssen-Cilag, UK
Dermatophyte skin infections caused by Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis and Epidermophyton floccosum: - dermatomycosis of smooth skin;
- inguinal athlete's foot; - epidermophytosis of the hands and feet; - skin candidiasis; - pityriasis versicolor; — seborrheic dermatitis caused by Pityrosporum ovale from 430
5.0 1 review
1508
- Like
- Write a review
Classification of antifungal drugs
Antifungal drugs are classified into:
- antifungals for systemic use: antibiotics: amphotericin B, griseofulvin;
- imidazole derivatives: ketoconazole;
- triazole derivatives: fluconazole, itraconazole, voriconazole, posaconazole;
- allylamines: terbinafine;
- echinocandins: anidulafungin, caspofungin, micafungin;
- antibiotics: nystatin, natamycin;
In addition, there are combination drugs containing an antifungal agent and a corticosteroid (clotrimazole + beclamethasone; isoconazole + diflucortolone; natamycin + neomycin + hydrocortisone), as well as: bifonazole + urea; clotrimazole + gentamicin + calendula extract + yarrow extract; clotrimazole + salicylic acid; miconazole + metronidazole.
Top 10 remedies for treating nail fungus.
May 21, 2022
7550
5
1
Content
- Exoderil
- Nizoral
- Lamisil
- Lotseril
- Clotrimazole
- Candide
- Mycoseptin
- Nitrofungin
- Terbinafine
- Mikoderil
Recently, more and more people are turning to dermatologists with a problem such as nail fungus. Symptoms of nail fungus include: peeling and loosening of the nail plate, unpleasant odor from feet and shoes, darkening of nails, etc.
Based on feedback from patients who had nail fungus, we have compiled a list of the most effective ointments and solutions, from which you can choose one product for your home medicine cabinet.
Read also Review of popular drugs against thrush The most popular drugs against thrush for internal and external use
Basics of antifungal treatment
The duration of treatment with antifungal drugs is always individual and usually depends on the type of mycosis, as well as the severity of the disease. The minimum course of treatment for vaginal candidiasis (thrush) is from 1 to 3 days. The maximum duration of treatment for nail mycoses (onychomycosis) is up to 6-12 months. On average, the duration of treatment for superficial mycoses is 14-30 days.
For mycoses of the nails and feet, topical use of antifungal drugs should be continued for another 2-3 weeks after the manifestations of the disease disappear in order to avoid exacerbation and return of the disease.
Publications
Currently, there is an increase in the number of inflammatory diseases of the nasal cavity and paranasal sinuses (SNS)[1]. At the same time, the phenomenon of a constant increase in the incidence of mycoses in this localization remains undeniable [2-6].
The introduction of new medical technologies into clinical practice (organ transplantation, high-dose immunosuppressive therapy, invasive diagnostic and therapeutic procedures), the HIV pandemic and the widespread use of antibacterial drugs have led to an increase in the population of immunocompromised patients with a high risk of developing invasive mycoses [7,8]. Not only have the number and severity of fungal infections in otorhinolaryngology increased, but there has also been a tendency to expand and change the spectrum of mycobiota [9]. When diagnosing mycoses, practitioners and laboratory workers often experience great difficulties [10,11].
The basis for the treatment of mycoses is the use of antifungal drugs (antimycotics). At the same time, various types of fungi have primary (natural) resistance to certain antimycotics, and the widespread use of these drugs for the prevention and treatment of mycoses in recent years has led to an increase in secondary resistance (primarily to fluconazole) [12]. For effective therapy of mycoses, a necessary condition is to carry out species identification and determine the sensitivity of pathogens to antifungal drugs [9-12]. Selection of a suitable antimycotic based on determining the sensitivity of the pathogen in vitro is more cost-effective than empirical replacement of one drug with another [9,11]. However, the lack of an accessible (simple to perform, economical and easily reproducible) method for determining the sensitivity of fungi does not allow these studies to be carried out in every clinical microbiology laboratory. Thus, studying the spectrum of pathogens of nasal and SNP mycoses and sensitivity to antifungal drugs is an urgent problem in otorhinolaryngology and medical mycology in general. Its solution in the future will make it possible to develop recommendations for rational empirical antimycotic therapy of fungal rhinosinusitis.
Unfortunately, in our republic there is a shortage of information on medical mycology in general. Many otorhinolaryngologists (and doctors of other specialties) associate the diagnosis of mycosis with the prescription of nystatin and fluconazole. For specific antifungal treatment, a number of effective drugs for both systemic and local use are currently used. Polyene antibiotics were the first to be used in mycology nystatin, natamycin, amphotericin B. The first two drugs are not used for systemic therapy due to low bioavailability (3-5%). Amphotericin B has been used for more than 40 years and has not lost its importance in the treatment of deep mycoses. Its main advantage is the widest range of antifungal activity. However, its use is limited by the high toxicity of the drug and the lack of an oral form. An effective method to reduce the toxicity of amphotericin B is the use of its liposomal forms [13]. The drug Ambizon is now registered in Russia, but its significant disadvantage is its high price. The introduction of triazoles (itraconazole, fluconazole) can rightly be called a revolution in the treatment of mycoses. For the first time, safe treatment and prevention of a number of deep mycoses became possible. (It should be noted that the first of the proposed “systemic” azoles - ketoconazole - after the introduction of itraconazole into clinical practice, practically lost its significance due to high toxicity and is now more often used topically). Sometimes attending physicians get the impression of the clinical equivalence, and even identity, of these drugs. However, itraconazole and fluconazole differ from each other in their main characteristics and have their own indications and prescription features.
The mechanism of action of azoles is based on inhibition of ergosterol synthesis due to the effect on the cytochrome P-450 system-dependent enzyme 14a-demethylase, which causes a fungicidal effect. Fluconazole is most active against most yeast fungi: causative agents of candidiasis (C. albicans, C. parapsilosis, C. tropicalis, C. lusitaniae, etc.), cryptococcus and coccidioides, but is inactive against molds and some yeast fungi (C. krusei, C.glabrata, C.norvegensis), which are often causative agents of deep mycoses, especially in the group of immunocompromised patients. In the last decade, the problem of secondary resistance of Candida spp has become quite acute. to fluconazole due to its widespread and unsystematic use [8,12]. Of the two drugs under consideration, itraconazole has the widest spectrum of action (yeast + mold) in vitro and in vivo. The spectrum of action of itraconazole includes Candida spp., Cryptococcus neoformans, Aspergillus spp., dermatophytes, Malassezia furfur, Dematiaceae fungi, Pseudallescheria boydii and all dimorphic pathogens of mycoses. Resistance to itraconazole develops extremely slowly.
Fluconazole and itraconazole are well absorbed from the gastrointestinal tract. At the same time, for the absorption of itraconazole, a sufficient level of acidity in the stomach is necessary, since, when reacting with hydrochloric acid, it is converted into highly soluble hydrochlorides. The bioavailability of itraconazole administered in capsule form is greater when taken with food. When taken orally, azoles should be taken with a sufficient amount of water. If there is low acidity in the stomach, these drugs are recommended to be taken with drinks that have an acidic reaction (for example, cola).
During treatment with systemic azoles, terfenadine and astemizole should not be taken, and when treated with itraconazole, lovastatin and simvastatin should not be taken. Peak concentrations in the blood of fluconazole are reached after 1-2 hours, itraconazole - after 2-4 hours. Itraconazole, being highly lipophilic, is distributed mainly into organs and tissues with a high fat content: liver, kidneys, greater omentum. It can accumulate in tissues that are particularly susceptible to fungal infection, such as skin (including the epidermis), nail plates, lung tissue, and the mucous membrane of the upper respiratory tract, where its concentrations are almost 7 times higher than in plasma. In inflammatory exudates, itraconazole levels are 3.5 times higher than plasma levels. At the same time, itraconazole practically does not penetrate into “aqueous” media - saliva, intraocular fluid, CSF. Neutropenic patients and those with AIDS may require an increase in dose [7,8].
For all systemic azoles, the most common side effects are from the gastrointestinal tract (abdominal pain, loss of appetite, nausea, vomiting, diarrhea, constipation), liver (increased transaminase activity, cholestatic jaundice). According to the literature, the incidence of adverse reactions to itraconazole is 18-25% [7,8,14]. Drugs such as griseofulvin and terbinafine are effective only for dermatophytosis.
Recently, the newest antimycotics have been registered in Belarus: voriconazole (triazoles) and caspofungin (echinocandins), characterized by a wide spectrum of action [13]. They are already successfully used in oncohematology hospitals in the republic, but wider implementation into practice is limited by the high cost of the drugs.
Thus, when choosing a drug for systemic antimycotic therapy (AMT) of chronic fungal rhinosinusitis, one should be guided by the following criteria: • action on the most likely causative agents of rhinosinusitis • good tolerability • bioavailability and penetration into the lesion (VDP, SNP) • affordable cost of the course of treatment (pharmacoeconomic indicators).
In this regard, the purpose of our work was to study the spectrum of mycobiota in chronic fungal sinusitis and the level of resistance to the main antifungal drugs for rational therapy, as well as to evaluate the effectiveness of itraconazole in this pathology.
Materials and methods.
The study included 35 patients who were treated at the ENT clinic of the Gomel State Medical University in 2006-2008. Of these, 12 (34.3) were men, 23 (65.7) were women, aged from 18 to 75 years (average age – 35+_7.1 years). In all cases, the fungal or fungal-bacterial etiology of sinusitis was confirmed. The classification of fungal rhinosinusitis was used in the work (Lopatin A.S., 1999, 2002; de Shazo RD et al., 1998) [3,5,6]: acute (fulminant) invasive rhinosinusitis - rhinocerebral mucorosis chronic fungal invasive rhinosinusitis non-invasive sinusitis (SNP mycetoma) allergic (eosinophilic) fungal rhinosinusitis
Diagnostics was based on a set of methods: ENT examination, optical rhinoscopy, X-ray examination (computer and magnetic resonance imaging), histological, immunological and, especially important, microbiological examination. Material was collected from the ED during puncture or intraoperatively before the start of antibacterial and antimycotic therapy. Transportation was carried out for 2-4 hours in test tubes on Amies carbon medium (Hema-Medica LLC, India). Identification, determination of the sensitivity of fungi and analysis of the data obtained were carried out using a miniAPI microbiological analyzer from bioMerieux (France). Cultures that have no clinical significance were excluded. Determination of sensitivity to antifungal drugs (amphotericin B, fluconazole, itraconazole and voriconazole) was carried out on strips (ATB FUNGUS-3) from bioMerieux (France) in a semi-liquid medium adapted to the requirements of the standard dilution method of the Clinical Laboratory Standards Institute (CLSI) - NCCLS M- 44, USA. To control the quality of susceptibility testing, control strains of the American Collection of Microorganisms (ATCC) were used.
Treatment included a whole range of methods: surgery, antimycotic and antibacterial therapy, mucolytics, antihistamines, immunocorrection, physiotherapeutic treatment. When carrying out systemic AMT, itraconazole ( Mikotrox ) was used in 100 mg capsules, produced by Pharmakar (Palestine-Germany). The duration of dynamic observation of the group of patients ranged from 6 months to 2 years with a frequency of 2 weeks, 1-3 months, then 2 times a year. Evaluation of the clinical effectiveness of treatment was carried out according to the following criteria : • cure - complete disappearance of sinusitis symptoms at the end of treatment, absence of exacerbations during the year; • improvement (remission) – disappearance/reduction of sinusitis symptoms at the end of treatment; • lack of effect – persistence of the main symptoms of the disease after the end of the course of treatment; • relapse – leveling of all initial symptoms by the end of treatment with their subsequent appearance within 12 months.
Criteria for mycological effectiveness: eradication - complete absence of fungi upon microscopy and 3-fold cultural examination of nasal swabs and SNP; lack of eradication - isolation of at least one colony of micromycetes. An analysis of adverse reactions was carried out in all patients, regardless of the duration of use of itraconazole (monitoring of CBC, BAC monthly).
Results and discussion.
According to our data, the most common form of fungal sinusitis was SNP mycetoma – 25 people. (71.4%). At the same time, mycetomas were more often localized in the maxillary sinus (MS) - 21 cases (84%), MSP+ cells of the ethmoidal labyrinth (LM) - 4 cases (16%). Chronic invasive form of fungal sinusitis was noted in 8 (22.9%) cases. Allergic fungal rhinosinusitis – in 2 cases (5.7%). In our studies, microbiological confirmation of the mycotic nature of sinusitis was noted in 24 cases (68.5%) (Fig. 1, 2), histological confirmation - in 8 (23%), mycological + histological - 2 (5.7%). In 1 (2.8%) case, the diagnosis of chronic invasive fungal rhinosinusitis was made only clinically (ex uvantibus).
Rice. 1 Growth of Aspergillus fumigatus colonies Fig. 2 Growth of Aspergillus fumigatus colonies on blood agar. on Sabouraud Wednesday.
When analyzing the results of mycological research, we found that in fungal rhinosinusitis, the mycelial (mold) mycobiota is of greatest importance: Aspergillus spp. (fumigatus, niger, flavus) - 18 (52.9%), Penicillium spp. 6 (17.6%), Mucor 4 (11.8%), Alternaria 1 (2.9%); Yeast micromycetes are much less common: C. albicans - 3 cases (8.8%) and C. non-albicans (krusei) - 2 cases (5.9%) (Fig. 3).
Rice. 3. Spectrum of mycobiota of SNP in chronic rhinosinusitis
A summary assessment of the sensitivity to antifungal drugs of the isolated 26 strains of micromycetes is presented in Fig. 4. It should be noted that all molds (100%) (Aspergillus, Penicillium, Alternaria) and C. non-albicans are resistant to fluconazole. C. albicans remains highly sensitive to fluconazole (100%). Resistance to itraconazole was detected in C. krusei in 1 case (2.9%). Unfortunately, it was not possible to isolate fungi of the genus Mucor by cultural method; therefore, we did not obtain data on their antimycotic resistance. Thus, based on the results obtained on the antimycotic sensitivity of the isolated strains, the drugs of choice for the treatment of fungal rhinosinusitis are itraconazole, amphotericin B, and voriconazole. The prescription of fluconazole is justified only if the etiological role of C. albicans is confirmed.
Fig. 4 Total antimycotic sensitivity of micromycetes isolated from SNP.
But even sensitivity to a drug in vitro does not mean the effectiveness of therapy, since when choosing a drug it is necessary to take into account the pharmacokinetics and localization of the pathological focus, the presence of severe concomitant pathology and immunodeficiency. The VCP is a closed cavity, difficult to access for any systemic drug. Therefore, the local use of amphotericin B (solution in 5% glucose 250,000 units once a day) in the form of inhalation and rinsing of SNP is quite justified. In most cases of fungal sinusitis, a combination of surgical treatment with systemic and/or local antimicrobial therapy is necessary.
In the postoperative period, the sinuses were washed with a solution of amphotericin B and inhaled through the nose with the same drug (7-10 procedures). Cure was noted in 100% of cases. No relapses were observed, which confirms the opinion of other authors about the inappropriateness of prescribing systemic AMT for SNP mycetoma [2,3,5,6]. In our opinion, the prescription of systemic AMT (minimum course of 14 days) for sinus mycetoma (2 cases) was justified in the absence of confidence in the non-invasiveness of the process (no CT, MRI, intraoperative macropicture data).
In the case of the invasive form of mycotic sinusitis (8 patients), in 100% of cases, radical surgery was performed on the upper jaw, the ethmoidal labyrinth, followed by systemic and local antimicrobial therapy. The duration of AMT was determined individually - from 1 to 6 months. Remission was achieved in 91.7% of cases, relapse of sinusitis was noted in 16.7%. We adhered to conservative tactics for the treatment of allergic fungal rhinosinusitis: itraconazole 200 mg per day for 1 month, inhalations with amphotericin B, antihistamines, nasal corticosteroids. In both cases, remission was achieved; there were no exacerbations of sinusitis for 6 months (follow-up is ongoing). In all 12 cases (100%) of systemic therapy with itraconazole ( Mikotrox ) it was possible to achieve mycological effectiveness at the end of the course of treatment.
With topical use of amphotericin B, side effects are minimal - soreness and dryness in the throat (4 cases), nausea (1 case). It should be noted that itraconazole ( Mikotrox ) was well tolerated in 9 patients (75%); only 2 patients (16.7%) developed nausea and unstable stools in the first week of treatment, but these symptoms were leveled out by diet correction without reducing the dose of the drug. In 1 case (8.3%), the patient noted heaviness in the right hypochondrium and bitterness in the mouth after a month of treatment (daily dose 400 mg), while no deviations in general and biochemical blood tests were recorded (in particular AlT, AST). After reducing the daily dose of itraconazole to 200 mg and prescribing hepatoprotectors, side effects were stopped. In none of the 3 cases was complete discontinuation of the drug required. There were no allergic reactions.
Based on our research, we can conclude that itraconazole ( Mikotrox ) meets all of the above criteria for systemic AMT and is the drug of choice for chronic fungal rhinosinusitis.
We present a case from practice. Patient G., 75 years old, was admitted to the clinic with complaints of aching pain and swelling in the area of the right cheek, purulent, foul-smelling discharge from the right half of the nose. From the anamnesis it became known that she had been ill for about 3.5 months, was treated on an outpatient basis (antibiotic therapy, punctures and lavage of the right upper quadrant) with a slight temporary improvement. Due to the ineffectiveness of outpatient treatment and deterioration of her condition, she was referred to the ENT clinic. Objectively: the general condition is satisfactory, slight infiltration and pain on palpation in the area of the anterior wall of the right upper sinus, during rhinoscopy - curvature of the nasal septum to the right, purulent discharge in the upper sinus on the right, swelling and hyperemia of the mucous membrane of the upper sinus and upper sinus, teeth - dentures. The remaining ENT organs are without pathology. The radiograph of the sinus shows a total inhomogeneous darkening of the right upper sinus, the remaining sinuses are airy. Upon puncture of the intracranial cavity, liquid pus is found. Against the background of antibacterial and anti-inflammatory therapy, daily punctures and washing of the right upper quadrant, the patient notes a significant improvement - nasal discharge has stopped, pain in the right cheek area does not bother, but infiltration in the same area persists. The patient underwent a computed tomography scan of the facial skull - in the right upper jaw there is an inhomogeneous darkening, contents, with fragments of bone density, destruction along the lower wall (Fig. 5).
Rice. 5 CT picture of an invasive fungal infection of the right upper quadrant.
A clinical diagnosis was made: chronic purulent maxillary sinusitis on the right, exacerbation. Secondary neuralgia of the I-II branches of N.trigeminus on the right. Organic disease of the right upper quadrant? (adamantinomas of the upper jaw?). To clarify the diagnosis, a maxillary sinusotomy (MS) was performed according to Caldell-Luc on the right: polyposis-altered mucosa was found in the sinus, along the anterior and lower carious-altered wall there were caseous-necrotic masses with bone fragments. A radical operation was performed on the right upper quadrant, all pathological contents were carefully removed.
Pathohistological conclusion: osteomyelitis of the upper jaw, necrotic dendritis, Asp mycelium threads. fumigatus, there is no evidence for a neoplasm. Microbiological study: growth of Fusobacterium (anaerobes) 10´7 was obtained, S – metronidazole, clindamycin; No fungal growth was observed. The final diagnosis was made: Chronic invasive fungal-bacterial maxillary sinusitis on the right, exacerbation. Secondary neuralgia of the I-II branches of N.trigeminus on the right. In the postoperative period, she received clindamycin 300 mg and itraconazole 200 mg per day, daily rinsing of the upper respiratory tract with solutions of Miramiscin, amphotericin B, inhalation through the nose with amphotericin B. She was discharged with improvement, it was recommended to continue treatment on an outpatient basis: clindamycin 300 mg per day for 10 days, itraconazole 200 mg per day for 2 months. After 2 months, the patient is admitted again with a similar clinical picture of exacerbation of maxillary sinusitis on the right. As it turned out later, she did not receive outpatient treatment (for social reasons). Objectively: with optical rhinoscopy, there is scanty purulent discharge in the ONH and the upper quadrant on the right, the artificial anastomosis is freely passable, there are black fungal masses at the bottom of the upper quadrant. Repeated microbiological examination revealed no growth. Conservative treatment was prescribed (daily rinsing with antiseptic solutions, amphotericin B, metronidazole 500 mg three times a day, itraconazole 200 mg per day). The dynamics were positive, after 10 days she was discharged with improvement, recommendations for outpatient treatment were given (continue taking itraconazole 200 mg per day for 1 month). During further dynamic observation for 3 months, no exacerbation of the disease was noted, and there were no complaints from the ENT organs.
Thus, this clinical example confirms the fact that fungal sinusitis does not have pathogonic symptoms, occurs under the guise of other diseases (in particular, neoplasms of the upper jaw), so their diagnosis is complex and requires an integrated approach. The validity and effectiveness of the use of itraconazole in invasive forms of fungal sinusitis is also clearly demonstrated.
Conclusions.
1. The dominant (85.3%) etiological significance in fungal rhinosinusitis is mold mycobiota (Aspergillus spp., Penicillium spp.). 2. For systemic antimycotic therapy of sinusitis, the drug of choice is itraconazole (Mikotrox®). Reserve drugs in modern conditions are voriconazole and amphotericin B. 3. Itraconazole (Mikotrox®) is an effective and safe drug in the treatment of sinusitis of mycotic etiology. 4. In order to increase the effectiveness of treatment of chronic fungal rhinosinusitis, the use of methods for identifying and determining the antimycotic sensitivity of mycotic pathogens is recommended.
Literature:
1. Semak, L.I. Sinusitis in the structure of hospital ENT pathology / L.I. Semak, A.R. Sakovich // New technologies in otorhinolaryngology: materials of the VI Congress of Otorhinolaryngologists of the Republic of Belarus, Grodno, May 15-16, 2008/ Grod. state honey. University, editorial board: A.Ch. Butzel [and others]. – Minsk, 2008. – P.131-132. 2. Zabolotny, D.I. The role of fungi in the pathology of the upper respiratory tract and ear / D.I. Zabolotny, I.S. Zaritskaya, O.G. Volskaya // Journal. ear nose. and throat Bol.-2002. – No. 5. – P.2 – 15. 3. Lopatin, A.S. Fungal diseases of the paranasal sinuses / A.S. Lopatin // Russian rhinology. – 1999. – No. 1. – pp. 46 – 48. 4. Ponikau, JP The Diagnosis and Incidence of Allergic Fungal Sinusitis / JP Ponikau // Mayo Clinic Proceedings. – September., 1999. Vol. 74. – P.877-884. 5. deShazo, R.D. Fungal Sinusitis / R.D. deShazo, K. Chapin, R.E. Swain // New England J. of Med. – 1997. – Vol. 337. – P.254 – 259. 6. Metson, MD Fungal sinusitis // MD Metson, SN Mardon, B. Ralph // The Harvard medical school guide to healing your sinuses / MD Metson, SN Mardon, B. Ralph – New York, 2005. – P.153-157. 7. Klimko, N.N. Principles of treatment of mycoses / N.N. Klimko // Mycoses: diagnosis and treatment: a guide for doctors / N.N. Klimko. – M., 2007. – Chapter 3 – P.35-87. 8. Sergeev, A.Yu. Modern antimycotics and principles of antifungal therapy / A.Yu. Sergeev, Yu.V. Sergeev // Fungal infections: a guide for doctors / A.Yu. Sergeev, Yu.V. Sergeev – M., 2004. – Chapter 2 – P.55-143. 9. Tastanbekova, L.K. On the issue of studying mold fungi in mycoses of the ENT organs / L.K. Tastanbekova // Bulletin of the Kazakh National Medical University. – 2004. – No. 1(23). – P.78-80. 10. Arabian, R.A. Diagnosis of mycoses/ R.A. Arabian, N.N. Klimko, N.V. Vasilyeva - St. Petersburg: Publishing house SPbMAPO, 2004. - 186 p. 11. Elinov, N.P. Aspergillus infection: approaches to diagnosis and treatment / N.P. Elinov, V.S. Mitrofanov, R.M. Chernopyatova // Problems of medical mycology. – 2002. – T.4.-No.1.-S.1-14. 12. Veselov, A.V. Epidemiology of candidiasis pathogens and their sensitivity to azoles: results of the ARTEMIS Disk study / A.V. Veselov [et al.] // Clinical microbiol. antimicrobial chemotherapy, 2005. - volume 7. - No. 1. – P.68-76. 13. Klimko, N.N. New drugs for the treatment of invasive mycoses / N.N. Klimko, A.V. Veselov // Clinical microbiology and antimicrobial. chemotherapy. – 2003. – No. 4. – T.5 – P.342-353. 14. Martin, MV The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a review/ MV Martin // J. Antimicrob. Chemother. – 1999. – Vol.44. – P. 429-437.
Redko D.D., Shlyaga I.D., Shevchenko N.I.
“Medical Panorama”, 2008 No. 9
Clotrimazole
This remedy for foot fungus is available in the form of a cream and ointment. The active substance clotrimazole penetrates deep into the nail plate, destroys the cellular structure of the pathogen, and stops the proliferation of the fungus. Clotrimazole is effective against all types of fungal infections. The course of treatment for mycosis with clotrimazole is three to four weeks. Contraindications include: first trimester of pregnancy, lactation, allergy to the components of the drug.
Clotrimazole-Teva
Merkle GmbH, Germany
Fungal infections of the skin and mucous membranes caused by dermatophytes, mold and yeast-like fungi, lichen versicolor, erythrasma, vulvovaginal candidiasis, trichomoniasis, mycoses complicated by secondary pyoderma.
from 16
5.0 1 review
578
- Like
- Write a review
Nitrofungin
This is one of the best remedies for fungus in this rating; the active ingredient of Nitrofungin is undecylenic acid. The drug acts not only as an antifungal agent, but also as a disinfectant and antiseptic. This medicine for fungus acts quickly: the symptoms of mycosis disappear, the fungus goes away. Nitrofungin should be used twice a day until the manifestations of the disease disappear completely. Contraindications: children under three years of age, pregnant and lactating women, allergies to the components of the drug.
Nitrofungin
IVEX Pharmaceuticals, Czech Republic
Fungal skin lesions: - rubrophytia;
- inguinal athlete's foot; - trichophytosis; - skin candidiasis; - mycoses of the feet; - fungal diseases of the external auditory canal. from 248
4.0 1 review
940
- Like
- Write a review
Lamisil
The active component of this remedy for foot fungus is terbinafine. “Lamisil” is effective in the early stages of mycosis; if it is an advanced case, it is recommended to use “Lamisil” in complex therapy or simply replace it with a stronger drug. Treatment with Lamisil lasts at least 2 weeks (the drug must be applied twice a day). Medicine for fungus is available in the form of a solution, gel, cream and spray. Due to its strong effect, Lamisil is not prescribed during pregnancy and breastfeeding, as well as under 12 years of age.
Lamisil dermgel
Novartis Pharma Services AG (Novartis Pharma), Switzerland
Fungal skin infections, incl.
mycoses of the feet (tinea pedis), inguinal athlete's foot (tinea cruris), fungal infections of the smooth skin of the body (tinea corporis) caused by dermatophytes, such as Trichophyton (including Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton verrucosum, Trichophyton violaceum), Microsporum canis and Epidermophyton floccosum; pityriasis versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (Malassezia furfur). from 446
363
- Like
- Write a review
Lotseril
The active substance of the drug for mycosis is amorolfine. This antifungal agent is a clear nail polish. "Loceryl" helps treat fungus directly on the surface of the nail. Amorolfine slows down the destruction of the nail by fungus, and with long-term use, it completely destroys the pathogen. The components of “Loceryl” do not have a negative effect on the body, even if you use this varnish for a long time. The course of treatment with Lotseril lasts one to two weeks, the only drawback is the high cost of the product.
Lotseril
Galderma, Switzerland
Loceryl is an antifungal drug for external use.
It has a fungistatic and fungicidal effect due to damage to the cytoplasmic membrane of the fungus by disrupting the biosynthesis of sterols. The content of ergosterol decreases, the content of atypical steric non-planar sterols accumulates. Has a wide spectrum of action. Highly active against both the most common and rare pathogens of fungal nail infections. from 826
5.0 3 reviews
2444
- Like
- Write a review
Terbinafine
This fungal remedy is available in the form of a solution, spray, cream and ointment. The active substance of the drug is terbinafine, which inhibits the proliferation of the fungus, destroys its cellular structure and ultimately completely destroys it. For one to two weeks, Terbinafine is applied 1-2 times a day. Contraindications include: pregnancy, lactation, age under three years, renal failure, cancer.
Terbinafine
Vertex CJSC, Russia
Prevention and treatment of fungus, fungal infections of the skin, including mycoses of the feet (“fungus” of the foot), inguinal athlete’s foot (tinea cruris), fungal infections of the smooth skin of the body (tinea corporis), caused by dermatophytes such as Trichophyton (incl. T. rubrum, T. mentagrophytes, T. verrucosum, T. violaceum), Microsporum canis and Epidermophyton floccosum;
yeast infections of the skin, mainly those caused by the genus Candida (for example, Candida albicans), in particular diaper rash; Versicolor versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (also known as Malassezia furfur). from 38
975
- Like
- Write a review
Mycoseptin
This is an inexpensive drug against foot fungus, and very effective. The active ingredients of the product are undecylenic acid and its salt. "Mikoseptin" destroys fungal cells and is a powerful antiseptic. The ointment relieves itching, redness and burning. "Mikoseptin" is used in a course (four to six weeks). The drug should not be prescribed to children under 2 years of age or if they are allergic to the components of the ointment.
Mycoseptin
Mikoseptin is an ointment for topical use with an antifungal effect.
Indications for the use of Mikoseptin ointment are: superficial mycoses of the skin caused by microorganisms sensitive to the active ingredients of the drug, in particular dermatomycosis of smooth skin, inguinal dermatomycosis, dermatomycosis of the feet, interdigital dermatomycosis, including mixed candidal and bacterial infections. from 321
612
- Like
- Write a review