Amizon® for children
Recommendations for use
Dosage regimen
For adults, the maximum single dose is 1 g and the maximum daily dose is 2 g. The recommended course of treatment, depending on the severity and etiology of the disease, is from 5 to 7 days. If the condition worsens or does not improve within 3 days, you should consult a doctor.
Children from 6 to 12 years of age are prescribed Amizon® tablets 0.125 g.
Adults and children over 12 years of age are prescribed Amizon® tablets 0.25 g.
Flu and other respiratory viral infections.
Treatment.
Adults and children over 12 years of age are prescribed 1 tablet (0.25 g) 3 times a day for 7 days.
Children from 6 to 12 years old are prescribed 1 tablet (0.125 g) 3 times a day for 7 days.
Prevention.
Children 6-12 years old are prescribed 1 tablet (0.125 g) every other day for 3 weeks.
Children from 12 to 16 years old are prescribed 1 tablet (0.25 g) every other day for 3 weeks.
Adults and children over 16 years of age are prescribed 1 tablet (0.25 g) per day for 3 days, then 1 tablet (0.25 g) per day every other day for 2 weeks.
Treatment of measles, rubella, chickenpox.
Children 6-7 years old – 1 tablet (0.125 g) 3 times a day.
Children 8-12 years old – 1 tablet (0.125 g) 4 times a day.
Children 13-14 years old – 1 tablet (0.25 g) 3 times a day.
Children 14-16 years old – 1 tablet (0.25 g) 4 times a day.
Adults and children over 16 years of age – 2 tablets (0.25 g) 3 times a day.
Treatment of mumps infection.
Adults and children over 14 years old: 1 tablet (0.25 g) 4 times a day for moderate severity of the disease and 2 tablets (0.25 g) 3 times a day for severe disease for 7 days.
Children 12-14 years old: 1 tablet (0.25 g) 3 times a day for 7 days.
In the complex therapy of pneumonia.
Adults – 1 tablet (0.25 g) 3 times a day for 10-15 days.
In complex therapy of angina.
Adults – 1 tablet (0.25 g) 3 times a day for 5 days with moderate severity of the disease; 2 tablets (0.25 g) 3 times a day for 7 days for severe disease.
Method and route of administration
Take orally after meals, without chewing.
Frequency of use with indication of time of administration
The frequency of use depends on the severity and etiology of the disease.
Duration of treatment
The recommended course of treatment, depending on the severity and etiology of the disease, is from 5 to 7 days.
Measures to be taken in case of overdose
No cases of overdose with Amizon® have been reported.
Symptoms: brown discoloration of mucous membranes, vomiting, abdominal pain and diarrhea. The development of edema, erythema, acne-like and bullous rashes, and fever may occur.
Emergency procedures: gastric lavage, symptomatic treatment.
Recommendations for seeking advice from a healthcare professional to explain how to use the drug
Amizon tablet film 250 mg x20
Amizon tablet film 250 mg x20
ATX code: J05AX (Other antivirals)
Active substance: enisamium iodide (enisamium iodide) Rec.INN registered by WHO
Dosage form
AMIZON®
tab. 250 mg: 10 or 20 pcs.reg. No.: LP-000230 dated 02/16/11 - Valid
Release form, composition and packaging
Tablets 1 tab.
methylbenzylcarbamidopyridinium iodide 250 mg Clinical and pharmacological group: Antiviral and immunomodulatory drug. Interferon synthesis inducer Pharmacotherapeutic group: Antiviral immunostimulating agent
Pharmachologic effect ,
Pharmacokinetics,
Indications
Treatment of influenza and other acute respiratory viral infections, including as part of complex therapy.
ICD-10 codes
ICD-10 code Indication
J06.9 Acute upper respiratory tract infection, unspecified
J10 Influenza caused by an identified influenza virus, dosage regimen
Orally after meals, without chewing, 0.5 g 3 times a day. The maximum single dose is 1 g, daily dose is 2 g. The recommended course of treatment is from 5 to 7 days.
Side effect
Bitterness in the mouth, heartburn, burning sensation in the throat, slight swelling of the oral mucosa, which does not require additional treatment or discontinuation of the drug. The drug is well tolerated; according to clinical studies, the incidence of adverse reactions is 6%.
Contraindications for use
Hypersensitivity to any of the components of the drug. Lactase deficiency, lactose intolerance, glucose-galactose malabsorption. Children under 18 years of age, pregnancy, lactation period.
Use during pregnancy and breastfeeding It is not recommended for use during pregnancy and breastfeeding.
Use in children Contraindicated: children under 18 years of age.
special instructions
Taking Amizon® does not affect the ability to drive vehicles and operate various mechanical equipment.
Overdose
In case of an overdose of the drug, the side effects described in the corresponding section may increase. When they appear, gastric lavage and symptomatic treatment are performed.
Drug interactions
Interactions with other drugs have not been sufficiently studied.
Storage conditions and periods
In a dry place, protected from light, at a temperature not exceeding 25°C. Keep out of the reach of children.
Shelf life: 3 years. Do not use after the expiration date stated on the packaging.
Conditions for dispensing from pharmacies The drug is dispensed with a prescription.
Diagnosis and treatment of ARVI: features of the drug Amizon
The prevalence of acute respiratory viral infections and the characteristics of symptoms are due to a significant variety of pathogens - influenza (A, B, C), parainfluenza, adenovirus, respiratory syncytial (RS), rhinovirus, enterovirus infections. Each ARVI has several types, and therefore, having had, for example, type A influenza, you can get sick again with the same disease, but this time type B [4–8]. Influenza is an acute disease with a short incubation period, sudden onset and cyclic course, which is characterized by severe toxicosis and damage to the upper respiratory tract and lungs. Influenza is characterized by severe intoxication: chills or chilliness, severe headache, muscle aches, sometimes in joints, pain when moving the eyeballs or when pressing on them, photophobia, lacrimation, severe weakness and fatigue, lethargy; these symptoms dominate the catarrhal syndrome on the first day of the disease. Already in the first hours of the disease, body temperature reaches a maximum of 39–40°C. The level of fever reflects the degree of intoxication, but in general these concepts cannot be identified. The temperature reaction to influenza is characterized by severity and relative short duration. The fever lasts for influenza A from 2 to 5 days, for influenza B it lasts a little longer, and then the temperature decreases through accelerated lysis. In 10–15% of patients, fever has a two-wave nature, which is associated with complications caused by bacterial flora or exacerbation of chronic diseases [9–17].
Parainfluenza is an anthroponotic acute viral disease that affects the upper respiratory tract and occurs with a syndrome of mild intoxication. With parainfluenza, the larynx is primarily affected (laryngitis and/or laryngotracheitis occurs), and then the bronchi (bronchitis and/or bronchiolitis) and, somewhat less frequently, the nasal mucosa (rhinitis). An increase in the incidence of parainfluenza is usually observed in the spring and autumn, but cases of the disease are observed year-round. Susceptibility to parainfluenza is general (both adults and children become infected). The disease begins with damage to the mucous membrane of the upper respiratory tract: all patients complain of hoarseness or hoarseness of voice (in some - even complete aphonia), sore or sore throat, cough (initially dry, then turning into wet with the release of serous sputum; if associated bacterial infection, purulent sputum begins to be released). Body temperature during parainfluenza is usually low (in adults no more than 38°C, in children it can be higher) or normal. During the illness, an infectious syndrome develops, and after an illness, a post-infectious asthenic syndrome develops: weakness, fatigue, headaches and muscle pain. The duration of the disease, if there are no complications, is on average 5–7 days. The cough may persist for up to 2 weeks. and longer.
Differential diagnosis of parainfluenza is difficult. With parainfluenza, like with no other acute viral respiratory disease (AVRD), a comprehensive assessment of symptoms is important for making a diagnosis: their intensity, duration, dynamics of appearance, combination with each other [18, 19]. Adenoviral infection. ARVI of adenoviral etiology are characterized by severe catarrhal syndrome in the form of rhinitis, pharyngitis, tonsillitis, and less commonly, catarrhal conjunctivitis and symptoms of intoxication. The proportion of adenoviral diseases among acute respiratory infections in adults ranges from 2 to 15%. The incubation period is usually 5-6 days, less often - 9-11 days. The disease is characterized by an acute onset with a rise in body temperature and the appearance of symptoms of intoxication, but it can also begin gradually. A prodromal period lasting up to 3 days is observed in 30% of patients and is manifested by malaise, cough, runny nose, and sore throat. The clinical picture of the disease is characterized by the predominance of catarrhal symptoms over the symptoms of intoxication. The disease begins with increased body temperature, malaise, headache, chills, sore throat, runny nose with severe rhinorrhea, cough, and in some patients - pain in the eyes, photophobia, and lacrimation. Fever ranges from low-grade to high levels. The maximum rise in temperature in half of the patients is observed on the 2-3rd day of illness, but in some patients it may occur on the first day of illness. Chills or slight chills are often noted. The reduction in fever in half of the patients occurs lytically. Undulating fever (up to 2–3 waves) is rare. The duration of fever is from 1 to 15 days. Headache is observed in most patients; it is usually localized in the frontal region and is characterized by mild or moderate intensity. Dizziness may occur when suddenly getting out of bed and when walking. Muscle pain and body aches are observed in more than half of the patients and last for 3–4 days. Sometimes there is nausea and vomiting, which usually appears at the height of the fever. Vomiting can be repeated. Meningeal syndrome is rare and lasts 1–2 days.
Unlike influenza and other acute respiratory viral infections, with adenoviral infection, some patients experience liver enlargement. A feature of adenoviral infection is also the often observed systemic enlargement of lymph nodes, most often submandibular, cervical and axillary. Sometimes there are polymorphic skin rashes and gastrointestinal dysfunction. Catarrhal syndrome lasts 8–15 days. Most patients experience rhinitis with severe rhinorrhea, first with serous, then mucous discharge, which is always combined with damage to the pharynx. Patients often complain of sore throat and cough. There is moderate hyperemia of the mucous membrane of the palatine arches, uvula, tonsils and posterior pharyngeal wall. Hyperemia of the soft palate is less common than with influenza and parainfluenza. The tonsils are often enlarged; in some cases, a delicate filmy coating appears on their surface in the form of dotted (islet) or larger whitish overlays. Conjunctivitis, which in children is considered pathognomonic of adenoviral infection, is rarely observed in adults. In the blood there is normocytosis with a tendency to a band shift of neutrophils and a decrease in the number of eosinophils and lymphocytes. ESR is normal or slightly increased. The most common complication is pneumonia [20, 21].
Respiratory syncytial infection. An acute infectious disease caused by the PC virus is characterized by moderate symptoms of intoxication and predominantly damage to the lower respiratory tract. The share of this infection among acute respiratory viral infections in adults is 3–8%. The incubation period lasts from 3 to 5 days. Prodromal symptoms are malaise, mild headache, cough, runny nose. The onset of the disease is most often acute. Intoxication syndrome is characterized by moderate symptoms and lasts from 1 to 7 days. The onset of the disease in half of the patients is manifested by chills, fever, headache, aches and a feeling of weakness. Moderate headaches are most often localized in the frontotemporal region, less often in the occipital region. Vomiting, nausea, and dizziness are usually observed in the first days of illness in a small number of patients. Occasionally, severe manifestations of intoxication with short-term loss of consciousness, convulsions, and meningeal phenomena may occur. A small number of patients have afebrile forms of the disease. Hemorrhagic phenomena during PC infection are rare and are manifested mainly by nosebleeds and hemorrhages on the mucous membrane of the soft palate. Catarrhal symptoms during PC infection in an adult are quite scanty, their duration is 4–6 days. Changes in the respiratory system consist of symptoms of damage to the upper and lower respiratory tract. The phenomena of bronchitis with an obstructive component are observed in 10% of patients. In the blood, eosinophilia and a neutrophil shift to the left are observed with a normal number of leukocytes (in uncomplicated forms of the disease). The most common complications of PC infection are pneumonia and sinusitis [20–23].
Rhinovirus infection. The incubation period of the disease is from 1 to 6 days. The onset is often acute, sometimes gradual. There are no prodromal symptoms. The first symptoms of mild intoxication are: malaise, “chilling”, nagging muscle pain, heaviness in the head, a slight increase in body temperature. Simultaneously with the symptoms of intoxication, catarrhal symptoms appear: sneezing, a feeling of rawness or sore throat. Rhinitis develops already in the first hours of the disease. Initially, nasal congestion and difficulty in nasal breathing are noted. After a few hours, mucous discharge appears from the nose, sometimes copious, with a watery consistency. After 1 day, the discharge becomes thicker, serous-mucous. Later, with the addition of bacterial flora, they acquire a mucopurulent character. Hyperemia of the pharynx and posterior wall of the pharynx is slightly expressed, most often the process is limited to the arches. Sometimes there is moderate swelling of the mucous membrane and “graininess” of the soft palate. Conjunctivitis in most patients is manifested by swelling and injection of the vessels of the conjunctiva, often the sclera, as well as profuse lacrimation. Laryngitis is observed frequently, its degree is insignificant, the main manifestations are “coughing” and hoarseness of the voice. Tracheitis and bronchitis are not typical for rhinovirus infection. The intoxication syndrome is usually mild. The fever is most often low-grade and lasts from several hours to 2-3 days. In some patients, there is no increase in body temperature. Malaise, muscle pain, usually of a “pulling” nature, are mild or moderate. Hematological changes sometimes manifest as slight leukocytosis. Rhinovirus disease is one of the mildest acute respiratory viral infections, but 10–15% of patients develop bronchitis or pneumonia [20].
Most often, the diagnosis of ARVI is carried out on the basis of the clinical picture and taking into account the epidemic situation. For the etiological diagnosis of respiratory diseases of a viral, mycoplasma and bacterial nature, serological reactions in paired sera obtained at the onset of the disease and during the period of convalescence (with an interval of 8–12 days) are widely used. The detection of antibodies (usually hemagglutinating and complement-fixing) to a specific microbe in the first serum and their increase by 4 times or more in the second serve as evidence of past infection. The paired serum method is usually used for retrospective diagnosis of previous infection [19, 20]. Modern technologies for the treatment of ARVI, especially influenza, provide a complex of therapeutic measures and include specific antiviral etiotropic drugs, pathogenetic and symptomatic therapy, as well as measures that increase the body's defenses. It is necessary to take into account the timing of therapy, the etiology of the infection and the pathogenetic features of the disease. By adequately influencing certain parts of the pathogenesis of the infectious process caused by respiratory viruses, it is possible to achieve the activation of the body's defense mechanisms, optimizing the course of the infectious disease and preventing its complications. The course of ARVI can be complicated by the addition of a bacterial infection, and in patients with bronchopulmonary diseases it can lead to the development of complications, in especially severe cases - even death. In patients with exacerbation of chronic obstructive pulmonary disease, an increase in the number of neutrophils in bronchoalveolar secretions and a weakening of the function of alveolar macrophages, as well as a decrease in the level of total T lymphocytes, a deficiency of immunoglobulin (Ig) classes M and A, and a decrease in the number of B cells are observed [24]. In bronchial asthma, the level of secretory IgA and the content of T- and B-lymphocytes are significantly reduced, and there is a deficiency of T-helper cells of the 1st order, which is characterized by a decrease in the production of interferon (IFN)-γ [25].
The addition of bacterial microflora to ARVI always lengthens and aggravates the course of the disease. Therefore, at present, to prevent the development of bacterial complications in acute respiratory viral infections, from the very first day of the disease it is recommended to use drugs that have a rapid and effective antiviral and immunotropic effect. Currently, a promising direction is the introduction into practice of combination drugs that combine antiviral, anti-inflammatory and immunomodulatory effects. The drug Amizon (enisamium iodide) has immunomodulatory and anti-inflammatory properties, and also has a pronounced antioxidant and membrane-stabilizing effect. The drug is registered for the treatment of ARVI and influenza. It should be especially emphasized that Amizon has a hepatoprotective effect. According to its chemical structure, Amizon is a derivative of isonicotinic acid (N-methyl-4-benzylcarbamidopyridinium iodide). The drug was created at the Institute of Pharmacology and Toxicology of the Academy of Medical Sciences of Ukraine, underwent a full cycle of experimental and clinical studies and, according to the decision of the Pharmacological Committee of the Ministry of Health of Ukraine (protocol No. 8 of October 31, 1996), was approved for use as an antiviral, anti-inflammatory and antipyretic agent. It is very important that, unlike most anti-inflammatory drugs, Amizon does not have an irritating effect. Previously, it was believed that some of these properties (in particular, anti-inflammatory and immunomodulatory effects) were mutually exclusive and could not be characteristic of one drug. This is precisely the uniqueness of Amizon, which determines the unusually wide range of its therapeutic action, and therefore the possibility of use in clinical practice in the treatment of various diseases, mainly as a means of pathogenetic therapy. All these features of the mechanism of pharmacological action of Amizon were studied in detail on a number of experimental and clinical models at the Institute of Epidemiology and Infectious Diseases named after. L. V. Gromashevsky of the Academy of Medical Sciences of Ukraine and at Lugansk State Medical University. As a result of these studies, it was established that Amizon has clearly defined antiviral, interferonogenic, antioxidant properties, normalizes the level of prostaglandins and cyclic nucleotides (the ratio of cyclic adenosine monophosphate and cyclic guanosine monophosphate), as well as microhemocirculation, reduces the severity of sludge syndrome and perivascular edema, which also explains its anti-inflammatory effect [26–29]. It is the anti-inflammatory and antipyretic properties of Amizon, as well as its synergism in relation to antibacterial agents, that served as the basis for the use of this drug in the complex therapy of bacterial infections with the presence of a clearly defined local inflammatory focus - acute chronic tonsillitis and its exacerbations.
In special studies carried out at the Department of Environmental Genetics and Immunology of the Ukrainian Scientific Center for Medical Genetics of the Academy of Medical Sciences of Ukraine, it was established that Amizon not only does not have a mutagenic effect, but can also provide an antimutagenic effect under certain conditions. Thus, in frequently and long-term ill children and adolescents from environmentally unfavorable regions, who, in order to prevent recurrent acute respiratory viral infections and sore throats, for 3-4 months. took Amizon in the autumn-winter period, along with a 3-4-fold decrease in incidence compared to comparison groups, the frequency of cytogenetic disorders in peripheral blood lymphocytes significantly decreased [30]. Similar results were also obtained in relation to the liquidators of the accident at the Chernobyl nuclear power plant, to whom Amizon was prescribed as an analgesic and anti-inflammatory agent. It should be noted that with an initially reduced level of IFN-α in the blood serum, its content during Amizon therapy increased by 2.6–3.8 times, which was demonstrated in a group of children and adolescents suffering from frequent acute respiratory viral infections and repeated sore throats and living in ecologically unfavorable environment. To date, the effectiveness of the drug has been established for a wide range of viral and mixed viral-bacterial infections.
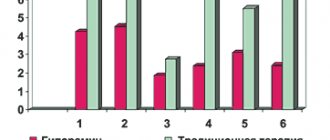
Antiviral effect of Amizon As an antiviral drug and an inducer of interferonogenesis, Amizon is a highly effective means of preventing and treating influenza and ARVI during periods of increased seasonal morbidity and during an epidemic. It is significant that even if influenza or ARVI occurred in people who received Amizon for prophylactic purposes, the diseases were relatively mild and did not lead to the development of complications (bronchitis, pneumonia, sinusitis), which were quite often observed in patients in the external and internal control groups. In a randomized epidemiological study conducted in closed groups (air defense units, military boarding school), patients in the main group (323 adults and 116 adolescents) received a combination of Amizon and ascorbic acid as a means of preventing influenza and ARVI, patients in the internal control group (384 adults and 105 adolescents) - bendazole in combination with ascorbic acid. At the same time, the multiplicity of differences in morbidity levels according to intensive indicators was 3.6 ± 0.2 times for adults, and 4.2 ± 0.15 times for adolescents with p < 0.001 [31].
Amizon is successfully used for the prevention and treatment of acute respiratory viral infections in children who are often and long-term ill. For children aged 5–12 years, the drug is prescribed at a dose of 0.125 g for 5–7 days in a row, with the prophylactic course repeated after 2 weeks. for 3–5 days. If prophylaxis with Amizon was started 7–10 days before the start of the seasonal period of increased incidence of ARVI, there was a significant (3–6 times) decrease in the frequency of repeated ARVI in the months of increased incidence (February - March and October - November). In addition, when ARVI occurred during the use of the drug, the disease was much milder and was not accompanied by the development of complications of bacterial etiology (otitis, bronchitis, pneumonia) [32].
Amizon is effective in the treatment of viral infections such as measles, rubella, chickenpox, and mumps. Its administration helps to quickly reduce elevated body temperature, eliminate other symptoms of intoxication, as well as reverse the development of local inflammatory processes. In patients with measles, when using the drug, the number of complications of inflammatory origin, in particular pneumonia and otitis, decreases; in adults, the disease occurs in a milder form, and the general condition improves. In patients with rubella, taking Amizon is accompanied by a decrease in the duration of the febrile period, rapid disappearance of symptoms of intoxication and lymphadenopathy. In case of chickenpox, the use of Amizon helps to reduce the duration of the febrile period and rashes, prevents pustulization of vesicles, and significantly reduces the severity of the exudative component. The use of Amizon allows to optimize the treatment of patients with mumps. Early administration of the drug not only accelerates the disappearance of the phenomena of intoxication and inflammation in the affected salivary glands, but also prevents the development of complications such as orchitis [29, 31, 33, 34]. Important results in the study of the antiviral activity of enisamium iodide (Amizon) against influenza viruses were obtained by leading scientists in the USA and reported at the III Antiviral Congress in Amsterdam (Netherlands) on October 12-14, 2014 (D. Boltz, X. Peng, M. Muzzio, v. Margitich).
Pharmacological effects of Amizon In in vivo experiments, it was found that Amizon has not only antiviral activity, but also anti-inflammatory, antipyretic, and analgesic properties. Unlike most anti-inflammatory drugs, Amizon does not have an irritating effect on the mucous membrane of the digestive tract, which allows it to be prescribed to patients with erosive and ulcerative pathology of the gastroduodenal zone. The analgesic effect of Amizon is realized through the reticular formation of the trunk and peripheral opioidergic mechanisms. The antipyretic properties of Amizon are due to the effect on the thermoregulatory centers of the brain. In terms of analgesic activity, the drug is not inferior to aminophenazone and metamizole sodium, and in terms of the severity of anti-inflammatory and antipyretic effects it is superior to salicylates, as well as phenylbutazone and ibuprofen [26].
Interferonogenic effect of Amizon Viral diseases are characterized by disruption of a number of parameters of the immune system, while the main regulators of immune reactions are cytokines. The cytokine-modulating activity of the drug Amizon during experimental infection in mice caused by the human influenza A virus (H3N2) was studied using prophylactic (24 hours before infection with the virus), therapeutic (24 hours after infection) and emergency prophylaxis regimens (in moment of infection). To characterize the cytokine-modulating activity of the drug Amizon, a method was used to assess the expression of cytokine genes by the production of messenger RNA [23]. Administration of the drug Amizon to uninfected mice caused activation in animals of the expression of genes for key cytokines - interleukins-1β, -2, -12. In patients with ARVI who received Amizon, on the 7th day of drug use there was a significant increase in the production of IFN-α and -γ compared to the baseline level and the placebo group [35]. Conclusion. Despite the fact that a whole system of consistent measures to prevent ARVI and influenza, including vaccination and the use of antiviral drugs, has long been developed, the problem of treating these diseases still remains relevant. This is explained primarily by high contagiousness, widespread infection, variability of the antigenic structure of viruses, drug resistance and the lack of specific prevention of ARVI. Immunization against influenza is carried out for adults and children, which is a very important measure, but does not exclude the possibility of exposure to other types of viral infections. Since no vaccines have been developed against ARVI of non-influenza etiology, preventive actions to prevent these infections are aimed primarily at stimulating the functioning of the immune system - this is the so-called nonspecific immunoprophylaxis. The drug Amizon has recently been actively used in the prevention of ARVI and influenza. Its use increases the body’s immune status during seasonal epidemics, which means the body’s resistance to the effects of viral agents increases. Amizon is effective against a wide range of viral pathogens, as well as combined viral-bacterial infections. The use of Amizon for prophylactic purposes during seasonal epidemics can reduce the incidence of ARVI by 3–5 times. In addition, patients with viral infections have a milder course of the disease and the absence of severe complications of the infection.
In order to prevent ARVI during epidemics, Amizon is prescribed to children from 6 to 12 years of age orally after meals, ½ tablet every other day for 3–4 weeks, and then at the same dosage 2 times a week. until the end of the disease outbreak. For older children (from 13 to 16 years old), it is recommended to take Amizon 1 tablet (0.25 mg) every other day for 2–3 weeks, then 2 times a week. within 1–2 months. For the prevention of ARVI, Amizon is prescribed to adults, 1 tablet daily for 3-5 days, then 1 tablet every other day for 2 weeks, and then 2 times a week. 0.25 mg until the end of the epidemic.
For emergency prevention (after direct contact with an infected person), it is advisable to take Amizon 1 tablet 3 times a day for 3-5 days, then 1 tablet a day until the danger of getting sick disappears. For emergency prevention, children are prescribed Amizon ½ tablet (0.0125 mg) 2 times / day in the first 3-4 days after unwanted contact, and then ½ tablet 1 time / day until the threat of infection disappears. Amizon has good anti-inflammatory and antipyretic properties, surpassing in these indicators some drugs from the group of non-steroidal anti-inflammatory drugs, but unlike many of them, it does not have an ulcerogenic effect, which allows its use in patients with existing gastrointestinal pathology. Amizon does not have a negative effect on hematopoietic functions and does not have nephrotoxic, teratogenic or carcinogenic effects. In terms of interferonogenic properties, it is noticeably superior to bendazole, is non-toxic, and does not cause pronounced side effects, which allows it to be widely used in the prevention of acute respiratory viral infections and in the treatment of children and the elderly. In addition, Amizon is effectively used in the prevention of ARVI. Of the contraindications of Amizon, only hypersensitivity to iodine preparations, children under 6 years of age and the first trimester of pregnancy should be noted [36–38].