Clinical effectiveness of the drug Reaferon-ES-Lipint in the treatment of influenza and ARVI
As an advertisement
Influenza and other acute respiratory diseases remain the most widespread infections. Influenza viruses affect various organs and systems and cause severe hypertoxic forms in 5% of patients. Mortality among hospitalized patients is 0.5–2.5%. Pneumonia, complicating influenza and ARVI, is reported in 2–17% of all patients with influenza and in 15–46% of hospitalized patients [5].
Violations of various parts of the immune system and nonspecific resistance contribute to the severe course of influenza [4, 5]. Great importance in the pathogenesis of influenza and acute respiratory viral diseases (ARVI) is attached to the development of a state of interferon deficiency [4]. Interferons (IFN-α/β and γ) are cytokines that regulate cell differentiation, growth and reproduction, therefore they are among the most important homeostatic agents and factors of nonspecific resistance of the body. The antiviral effect is expressed in IFN-α/β, and the immunoregulatory and antiproliferative effects are characteristic of IFN-γ [4].
The antiviral effect of interferon α-2b manifests itself during the period of virus reproduction through active inclusion in the metabolic processes of cells. Interferon, interacting with specific receptors on the surface of cells, initiates a number of intracellular changes, including the synthesis of specific cytokines and enzymes (2-5-adenylate synthetases and protein kinases), the action of which inhibits the formation of viral protein and viral ribonucleic acid in the cell.
The immunomodulatory effect of interferon α-2b is manifested in an increase in the phagocytic activity of macrophages, an increase in the specific cytotoxic effect of lymphocytes on target cells, a change in the quantitative and qualitative composition of secreted cytokines, a change in the functional activity of immunocompetent cells, a change in the production and secretion of intracellular proteins.
The purpose of this work was to evaluate the clinical effectiveness of Reaferon-ES-Lipint in the treatment of patients with influenza and ARVI.
Reaferon-EC-Lipint is a domestic preparation of recombinant human interferon α-2b in liposomal form. The drug has antiviral and immunomodulatory activity. Liposomes, unlike polymer drug delivery systems, are completely biodegradable and biocompatible, protect protein from the acidic contents of the stomach, ensure complete and rapid absorption through the intestine and long-term circulation of IFN in the blood with further induction of endogenous IFN. The drug Reaferon-ES-Lipint is easy to dose, available for use, and safe [1, 3].
Materials and research methods
Under observation were 45 patients of both sexes, aged 18–50 years, with symptoms of influenza and ARVI. In addition to the general clinical examination, immunofluorescence examination of smears of secretion from the lower nasal passages, serological and x-ray examination of patients were carried out. The results obtained were statistically processed.
Patients of the main group (n = 30) in addition to basic therapy received 500 thousand IU of the drug Reaferon-EC-Lipint orally twice a day for 3 days. Patients in the control group (n = 15) received only basic therapy drugs (Antigrippin, multivitamins, expectorants). The effectiveness of Reaferon-ES-Lipint was taken into account according to the following criteria: duration and intensity of the temperature reaction, duration of infectious intoxication (headache and muscle pain, malaise, weakness, loss of appetite), dynamics of the development of catarrhal symptoms (cough, runny nose, sore throat), frequency of complications .
Results and discussion
A comprehensive laboratory examination showed that in 35 patients the disease was caused by influenza A (H3N2) and A (H1N1) viruses, in 10 cases of acute respiratory viral infection of various etiologies.
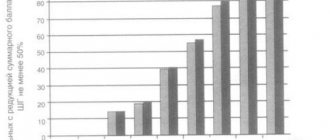
A clinical marker of the severity of infection is the severity of febrile reactions. With uncomplicated influenza among those taking Reaferon-EC-Lipint, 70% of patients had a body temperature that did not exceed 38.5 °C, which indicates a mild course of the disease, and only 10 people (30%) had a moderate course of influenza with a body temperature above 38. 6 °C. In the control group, against the background of basic therapy, 51.5% of patients had a fever above 38.6–39.0 °C, thus, in more than half of the patients in the control group the disease was moderate. The duration of fever in 81.8% of patients in the main group who received Reaferon-ES-Lipint did not exceed 2–3 days; with basic therapy, in 54.5% of patients the fever lasted 4–5 days. The inclusion of the drug Reaferon-EC-Lipint in the basic therapy regimen made it possible to reduce the severity and duration of the febrile period.
While taking Reaferon-ES-Lipint, symptoms of infectious intoxication in the form of headache, malaise, and weakness were relieved on average 1.2 days faster than in the control group (p < 0.05).
At the time of inclusion in the study, mucous, mucopurulent nasal discharge was recorded in 100% of patients in the main and control groups. This symptom resolved in a shorter period of time (0.5 days less) in patients of the main group than in the control group; the differences were not significant (p > 0.05). The cough symptom was recorded in the main group for 4.2 ± 0.25 days, which was significantly shorter by 1.5 days than in the comparison group - 5.7 ± 0.2 (p < 0.05). The symptom complex of tracheobronchitis (cough, voice changes, hard breathing, wheezing) was recorded in 5 patients of the main group and 4 control groups. The average duration of symptoms was 1 day shorter in patients of the main group (3.5 ± 0.25 days) than in the control group (4.5 ± 0.3 days) (p < 0.05).
Thus, while taking Reaferon-ES-Lipint, patients experienced catarrhal symptoms faster (on average 3.5–4.2 days); in the control group, these symptoms manifested themselves longer (4.5–5.7 days, p < 0 .05).
The overall average duration of one case of uncomplicated influenza when taking the drug Reaferon-ES-Lipinta was 1 day shorter (p < 0.05).
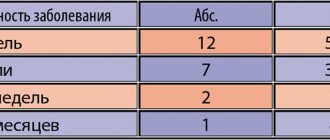
During the period of active observation, the development of complications was recorded in 13.3% of the main group and 33.3% of the control group (nosebleeds in 6 patients, pneumonia in 3 patients). The incidence of complicated influenza and acute respiratory viral infections was 2.5 times lower compared to the group of patients who received only basic therapy (p < 0.001).
Thus, the use of the drug Reaferon-EC-Lipint in patients with moderate forms of influenza and other acute respiratory viral infections led to a statistically significant decrease in the duration of objective signs of the syndrome of general infectious intoxication, productive cough and a decrease in the incidence of complications. The drug was well tolerated, the organoleptic qualities of the drug were satisfactory, and no adverse drug reactions were recorded.
The high clinical efficacy and safety of the drug Reaferon-ES-Lipint as part of complex therapy for influenza and other acute respiratory viral infections has been shown by other researchers [6]. The drug was successfully used for emergency prevention of influenza and other acute respiratory viral infections in children and adults during influenza epidemics or seasonal increases in morbidity [2, 3]. Our studies allow us to recommend the drug Reaferon-ES-Lipint for the treatment of acute respiratory infections.
conclusions
The inclusion of the drug Reaferon-ES-Lipint in the basic treatment regimen for influenza and ARVI improves the clinical effectiveness of treatment.
Literature
- Bazhutin N. B., Zolin V. V., Kolokoltsov A. A., Targonsky S. N. Prospects for the use of liposomal drugs in medical practice // Terra medica. 2003. No. 3 (31). pp. 3–6.
- Erofeeva M.K., Maksakova V.L., Kolyvanova I.L. et al. Reaferon-ES-Lipint as a means of emergency prevention of influenza-like viral diseases // Cytokines and inflammation. 2004. T. 2. No. 4. P. 44–47.
- Erofeeva M.K., Maksakova V.L., Pozdnyakova M.G., Kolyvanova I.L. Possibility of using liposomal alpha-2b interferon for the prevention of influenza and other acute respiratory viral infections // Issues of modern pediatrics. 2007. T. 6. No. 1. P. 42–46.
- Ershov F. I., Kiselev O. I. Interferons and their inducers (from molecules to drugs). M., 2005. 368 p.
- Isakov V. A. Clinical and pathogenetic aspects of severe influenza // Allergol. and immunol.//2002. Vol. 3, No. 1, pp. 136–144.
- Lobzin Yu. V., Lvov N. I., Kolokoltsov A. A. Clinical effectiveness of the drug “Reaferon-ES-Lipint liposomal” in the complex therapy of patients with influenza and other acute respiratory diseases when administered orally/Reaferon-ES-Lipint. Collection of articles and theses. Koltsovo, 2003. pp. 3–11.
V. A. Isakov*, Doctor of Medical Sciences, Professor V. Ya. Sergeeva* T. E. Efimova* I. V. Kabolova* S. N. Targonsky** O. N. Mukhina** M. G. Sharypova* *, 1
* Medical LLC, Veliky Novgorod ** Vector-Medica CJSC, Novosibirsk
1 Contact information
Reaferon-ES-Lipint® Lyophilisate, bottle, 6 pcs., 500,000 IU, for suspension preparation
Directions for use and doses
It is administered orally. Immediately before use, add 1-2 ml of distilled or cooled boiled water to the contents of the bottle. Shaking for 1-5 minutes should form a homogeneous suspension. For acute hepatitis B, the drug is taken 30 minutes before meals according to the following scheme:
- adults and school-age children - but 1 million IU 2 times a day for 10 days;
- children of preschool age (from 3 to 7 years) - 500 thousand IU 1 time / day for 10 days or. after control biochemical blood tests, for a longer period until complete clinical recovery.
For chronic hepatitis B in active and inactive replicative forms, as well as for chronic hepatitis B associated with glomerulonephritis, the drug is taken 30 minutes before meals according to the following scheme:
- adults and school-age children - 1 million IU 2 times/day for 10 days and then for 1 month - every other day, 1 time/day (at night);
- children of preschool age (from 3 to 7 years) - but 500 thousand IU 2 times / day for 10 days and then - 500 thousand IU for 1 month every other day, 1 time / day (at night).
When carrying out specific immunotherapy, the drug is taken in the morning, 30 minutes after meals. according to the following scheme:
- for allergic rhinoconctivitis in adults - 500 thousand IU daily for 10 days (course dose 5 million IU);
- for atonic bronchial asthma in adults - but 500 thousand IU 1 time / day for 10 days, and then 500 thousand IU every other day for 20 days. The total duration of treatment is 30 days.
For the prevention and treatment of influenza and ARVI, the drug is taken 30 minutes before meals:
- for prevention: adults and children over 15 years old - 500 thousand IU 1 time / day 2 times a week for 1 month during an increase in incidence; children from 3 to 15 years old, 250 thousand IU 1 time / day, 2 times a week for 1 month during an increase in incidence;
- for the treatment of influenza and ARVI: adults and children over 15 years old - 500 thousand IU daily 2 times a day for 3 days: children from 3 to 15 years old - 250 thousand IU daily 2 times a day for 3 days.
For complex therapy of urogenital infections in adults, the drug is taken 30 minutes before meals, 500 thousand IU daily, 2 times a day for 10 days. For complex therapy of tick-borne encephalitis, the drug is taken 30 minutes before meals:
- for febrile form: 500 thousand IU 2 times a day (morning and evening) for 7 days;
- for the meningeal form: 500 thousand IU 2 times a day (morning and evening) for 10 days.
For emergency prevention of tick-borne encephalitis, the drug is taken 30 minutes before meals, 500 thousand IU 2 times a day (morning and evening) for 5 days. Anti-tick immunoglobulin is administered intramuscularly once no later than the 4th day after the tick bite at a dose of 0.1 ml/kg.