Paroxetine - an antidepressant from hell?
Start
Paroxetine, also known by the trade names Aropax, Paxil, Pexeva, Seroxat, Sereupin and Brisdelle, was first marketed in 1992. Effective for major depression and various anxiety disorders, it has quickly gained a significant share of the antidepressant prescription market. However, by the late 1990s, paroxetine became frequently associated with serious drug interactions and side effects. When Paxil (paroxetine) was first approved, it was called "a much-needed and welcome addition to the antidepressant/hypnotic arsenal."
Mechanisms of action of paroxetine
In addition to being a serotonin reuptake inhibitor, it also had mild to moderate noradrenergic effects through norepinephrine reuptake inhibition (NRI or NARI) and could activate, which often helped depressed patients with lethargy.
Indications for the use of paroxetine
Paroxetine has been effective in the treatment of generalized anxiety, panic, post-traumatic stress, social phobia, premenstrual dysphoric disorder and obsessive-compulsive spectrum disorders
Side effects and complications from paroxetine therapy
Paroxetine has been reported to affect male fertility, birth defects, gestational hypertension, QT prolongation in infants, hyperprolactinemia, cognitive impairment in the elderly, autism, sexual side effects, weight gain and suicidality, aggression and akathisia in children and adolescents. Paroxetine has the highest known affinity for the serotonin transporter (0.13 nanomolar) of any antidepressant currently used.
Sexual disorders
Patients may be willing to tolerate some side effects, such as sexual dysfunction, early in treatment, but they are likely less willing to tolerate side effects that reduce their quality of life during ongoing treatment.
Paroxetine may cause cancer
By the early 1990s, animal studies showed that antidepressants increased the incidence and growth of breast cancer in mice. The researchers hypothesized that this increase in tumor incidence and growth may have been associated with inhibition of enzymes (eg, CPY450 2D6 isoenzyme) that are involved in the metabolism of carcinogens and estrogens, resulting in increased concentrations and serum levels of carcinogens and estrogens that are associated with breast cancer Another cause of tumorigenesis is increased inhibition of dopamine release in pituitary lactotrophs, leading to disinhibition. prolactin production and its increased level.
Between 2001 and 2006, several groups of researchers published case-control studies and reviews of antidepressant and breast cancer studies. Some have concluded that there is no association between antidepressant use and breast cancer, while others have concluded that there is an association and/or that a possible association between antidepressants and breast cancer risk has not been ruled out and more research is needed. In a 2006 study, Chien et al. concluded that there is limited evidence that ever use of antidepressants is associated with overall breast cancer risk. However, they found that SSRIs may increase the risk of progesterone receptor-negative (PR-), estrogen receptor-negative, and progesterone receptor-negative (ER+/PR-) tumors, although further research is needed to confirm this association. 33% (20 of 61) of studies reported a positive association between antidepressants and cancer. 67% (41/61) of studies reported no association or antiproliferative effect. It was concluded that preclinical and clinical data are mixed in demonstrating an association between antidepressant use and breast and ovarian cancer.
Paroxetine has the highest inhibition constant for the P450 2D6 isoenzyme of all antidepressants (Ki = 0.065–4.65 μmol). This high affinity explains its high inhibitory interaction profile with 2D6 substrates. The potent inhibition of 2D6 by paroxetine also means that there is significant inhibition of the metabolism of carcinogenic 2D6 substrates, implying an increased likelihood of tumorigenesis. Paroxetine was associated with a 620% increase in the risk of breast cancer in women who took it over a four-year period. A Canadian epidemiological study of antidepressant use and breast cancer found that paroxetine had an odds ratio (OR) for breast cancer in women treated for depression and/or anxiety over a four-year period of 7.2. This was several times higher OR than any other antidepressant or class of antidepressants in this study, including tricyclic antidepressants (TCAs), which were found to have a median OR of 2.0.
Through 2D6 inhibition, the metabolism of tamoxifen is inhibited, which increases the risk of death from breast cancer over a five-year period in women taking both drugs. Paroxetine is also a potent 3A4 inhibitor with multiple interactions with 3A4 substrate.
conclusions
1) Do not use paroxetine as first-line therapy in antidepressant-naïve patients with a family history of breast cancer; 2) If a woman with no history of breast cancer responds well to paroxetine, continue paroxetine; 3) If the woman has a strong family history of breast cancer (mother, sisters, grandmothers or aunts) or tests positive for BRCA1 or BRCA2, after consultation with a gynecologist, stop taking paroxetine and prescribe another drug; 4) Always gradually reduce the dose of paroxetine before stopping and/or cross-tap slowly when being treated with another drug
Clinical effectiveness of the drug Rexetine (paroxetine) for anxiety spectrum disorders
Rumyantseva G.M., Stepanov A.L., Levina T.M., State Scientific Center for Social and Forensic Psychiatry named after. V.P. Serbsky, Moscow
Introduction
Anxiety-phobic disorders are among the most common mental disorders encountered in general medical practice (A.B. Smulevich, 1999).
The higher prevalence of anxiety spectrum disorders among the population compared to depressive disorders was also revealed in large epidemiological studies conducted in the last decades of the last century (D. Regier et al., 1998).
Anxiety and depression, according to a number of scientists, are not independent diseases, but only stages in the development of a single affective pathology, in which anxiety is a more adaptive manifesting syndrome (H. van Praag, 2000).
In the structure of anxiety spectrum disorders, phobic, obsessive, hypochondriacal, compulsive and other symptoms that are part of the anxiety syndrome or comorbid disorders often occupy a significant place.
In the process of studying the contingent of people turning to private medical centers specializing in the treatment of borderline mental disorders, it was revealed that anxiety-phobic disorders occupy one of the first places among the reasons for seeking help (F40-F48 ICD-10).
At the same time, a certain specificity of the content of phobic manifestations was noted. Patients experience difficulties in expressing/verbalizing their fears, since their content could be morally condemned, incomprehensible to others, regarded as a sign of a serious and dangerous mental illness, for example, fear of harming a child or another loved one, fear of possible pregnancy and childbirth, fear of infection venereal disease, fear of homosexual attraction and/or the possibility of such contacts, fear of not being able to resist urination or defecation in a public place, etc.
As a rule, patients, suffering for a long time from their painful experiences, were afraid to go to medical institutions and hid their fears from others, often resorting to the help of psychics, removing the evil eye and damage, or religious rituals. In cases where anxiety became severe or comorbid disorders (somatoform and depressive) were added, they sought help in private medical centers, where, in their opinion, medical confidentiality is better maintained, anonymity is guaranteed and greater attention, support and care from the doctor are possible.
Such conditions are most often regarded as specific (isolated) phobias. However, at the time of contacting a specialist, the phobia, as a rule, is accompanied by a pronounced affect of anxiety and often comorbid depressive, somatoform manifestations, and panic disorder. This study is devoted to a clinical study of the effectiveness of treatment of these disorders with an antidepressant from the group of selective serotonin reuptake inhibitors (SSRIs) Rexetine.
Materials and methods
Under our supervision were 20 sick women aged 25-37 years, whose condition could be assessed within the framework of the ICD-10 rubric F40.2 - isolated phobias.
All patients had higher or incomplete higher education; 3 had two higher educations. 18 patients were married or living together; 13 of them had children.
At the time of treatment, patients complained of obsessive experiences of contrasting content: in 11 people it was the fear of harming a child or husband, which was usually accompanied by a fear of sharp, piercing objects.
In 7 of these patients, the primary phobia was supplemented by the fear of going crazy and/or, having lost control of oneself, committing a ridiculous action: screaming, hitting, losing consciousness and being left without help, not being able to hold back urination.
3 patients had an obsessive fear of sexual attraction to members of the same sex.
In 6 patients, it was the fear of pregnancy and the possibility of carrying it to term due to the “state of their health” and the consequences for the “mental usefulness” of the unborn child.
The onset of phobic disorders in all patients is between the ages of 20-27 years.
In a clinical study, it was determined that half of the premorbid patients were close to accentuated personalities with anxious and anancastic character traits, prone to increased anxiety, responsibility, hypernormativity, doubts and fears, mainly of hypochondriacal content. Typically, these patients showed a tendency to obsessive fears regarding their health already in childhood. As a rule, these features became sharper after emotional shocks (serious illness and death of a close relative or peer, alcoholism of one of the parents, situations where children witnessed an accident or were exposed to the threat of violence).
The remaining patients did not have pronounced character accentuations and were distinguished by activity, purposefulness and were socially successful.
Their fears arose against the background of a long-term psychologically traumatic situation (disharmony in family or partner relationships, conflict at work) or shortly after acute stressful experiences (attack and violence, death of the first child, forced abortion, miscarriage, breakup).
The aggravation of painful manifestations occurred gradually either due to the addition of obsessions of other (hypochondriacal) content (fear of madness, stroke/heart attack, cancer), or due to increased anxiety and the appearance of depressive syndromes.
Despite the complexity of the clinical picture, the diagnosis of a specific phobia always came first, since for a long time the disease was limited only to these symptoms. Were comorbid in
In 9 cases there was panic disorder (F41.0), in 11 cases there was mixed anxiety-depressive disorder (F41.2).
Panic disorder occurred at the height of obsessive fears (for example, harming a child, losing consciousness on the street, on the eve of medical examinations). The structure of panic disorder included pronounced fear, bodily tension, vegetative-vascular and somatic components. The frequency and severity of panic attacks were insignificant, the conditions did not require emergency medical care, and patients coped with panic symptoms on their own or with the help of loved ones. In all patients, sleep was disturbed, dreams acquired a painful, anxious, depressing character. Avoidance behavior developed: patients stopped visiting places where there were sharp and piercing objects, subways, elevators, closed spaces, and avoided air travel.
In cases of the development of mixed anxiety-depressive disorder, the patients' condition was characterized by increased generalized anxiety. At the same time, there were equally somatic and mental components of anxiety. Then a depressed mood followed with irritability, insomnia, anhedonia, decreased appetite, and progressive loss of body weight.
The Hamilton Anxiety and Depression Scale and the Sheehan Phobia Scale were used to examine the patients.
All patients were prescribed rexetine (paroxetine) at an initial dose of 20 mg. The dose was increased to 40 mg as indicated after 1 week. 3 patients and at the 4-5th week of treatment in another 5 patients. During treatment, the patients did not change their usual life schedule and did not lose their ability to work.
In the 1st week of treatment, all patients received benzodiazepines (alprazolam, clonazepam) to correct acute feelings of anxiety, restlessness and characteristic insomnia. The dose was reduced and benzodiazepines were discontinued gradually by the end of the 2nd week.
The total duration of treatment was 5 weeks.
Results and discussion
The most sensitive to the effects of rexetine was the anxiety symptom complex.
Thus, already in the 1st week of treatment, some patients began to notice a decrease in bodily tension and feelings of internal trembling, sweating, the intensity of the feeling of incomplete inspiration decreased, and there was a tendency to improve sleep.
During the 2nd week, the regression of anxiety was more significant: influxes of painful thoughts and images of the main phobic experience (fear of going crazy/losing control, causing harm, etc.) were less intense and more rare; daily mood swings became less severe; manifestations of dissatisfaction, hostility and irritation towards the immediate environment softened.
It should be noted that the therapeutic effect occurs quite quickly. The most pronounced decrease in the intensity of anxious experiences and improvement in the well-being of patients was observed during the first 4 weeks. therapy with rexetine. Subsequently, the positive dynamics slowed down somewhat and became more gradual.
A decrease in the intensity and burden of the “mental chewing gum” followed after 2-3 weeks. along with a decrease in overall affective tension (sensual charge of experiences). Normalization of well-being to the initial, pre-painful state was observed starting from the 3-4th week of treatment.
Depressive symptoms began to reverse most significantly after 3 weeks. treatment: at first, patients reported a decrease in the constant feeling of weakness and fatigue, an increase in strength and the emergence of desires and interest in life. At this time, emotional revival and a decrease in motor and mental retardation were observed. There was also a reduction in anhedonic complaints (lack of pleasure from communication, sex, food, reading, creative hobbies, work; a feeling of apathy). By this time, the vegetative stabilizing effect of the drug appeared.
Patients (4 people) noted a decrease in the frequency of gastrointestinal discomfort: senestalgia in the intestinal area, belching with air, gas formation, morning diarrhea and the urge to urinate before leaving the house.
The dynamics of essential phobic manifestations should be especially emphasized.
If the first 2 weeks. the improvement was mainly due to the reduction of the anxiety symptom complex, then in the 3rd week of therapy a decrease in the frequency of occurrence of phobic experiences was noted. Patients could not remember their fears for most of the day; they appeared sporadically in the morning and evening hours. The strength of obsessive fears also decreased; they arose “on the periphery of consciousness,” without completely absorbing the patients’ attention. The most intense reduction was observed in the fear of madness, which was most closely associated with anxious affect. The lowest dynamics were noted for pregnancy phobia. The fear of causing harm to loved ones occupies an intermediate position in terms of therapeutic lability.
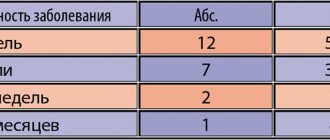
An almost complete reduction of phobias was achieved in 5 patients in whom the structure of the syndrome was complicated by a mixed anxiety-depressive disorder. In 12 patients, it was noted that the remaining phobic experiences were not emotionally saturated and were a “pale shadow” of those that existed before treatment. In 3 patients, despite the positive dynamics of painful symptoms, phobias remained unchanged, only their affective intensity decreased.
By the 4-5th week of treatment, 5 patients began to experience a “freezing” of the clinical picture - the absence of further positive dynamics, which required increasing the dose of the drug to 40 mg per day.
The most resistant to therapy remained the personal characteristics of patients: uncertainty, resentment, experience of one’s own imperfection, high personal anxiety. These experiences in most patients were long-term, latent, associated with the personality structure, and the patients adapted to them.
It should be noted that the majority of the patients we treated did not note the effect of excessive activation, “excitement,” restlessness and increased anxiety in the 1st week of treatment, characteristic of some SSRIs (fluoxetine, Paxil).
In the 1st week of treatment, 8 patients still reported increased difficulty falling asleep, frequent awakenings and greater irritability, which required the prescription of tranquilizers.
In 5 patients during the 1st and 2nd weeks. treatment, an increase in the phenomenon of sweating and a feeling of heat was noted, especially with excitement and when staying in stuffy rooms.
In no case did we observe neuroleptic symptoms (hypertonicity and hyperkinesis of the facial muscles and tongue, tremor, etc.) and significant phenomena of behavioral toxicity, as well as dysuria (given the fact that the sample of patients was female). However, 1 patient with a significant decrease in body weight (46 kg) and dysmenorrhea required a reduction in the dose of rexetine to 10 mg per day for 2 weeks. at the beginning of treatment.
Conclusion
The use of rexetine in dosages of 20-40 mg for isolated phobias with comorbid panic disorder (F41.0) and mixed anxiety-depressive disorder (F41.2) revealed the high effectiveness of the drug in relieving symptoms of anxiety and depression, as well as a pronounced antiphobic effect of paroxetine, which was shown in earlier works (S.N. Mosolov et al.).
The drug significantly alleviates the condition of patients, reduces the intensity and frequency of obsessive and phobic manifestations and improves the quality of life of patients. The distinct anxiolytic properties of rexetine with good tolerability make it possible to recommend it for use in outpatient practice for the treatment of isolated phobias with comorbid manifestations of panic disorder and mixed anxiety-depressive states.
References 1. Smulevich A.B., Ivanov S.V., Dubnitskaya E.B., Drobizhev M.Yu. // Smulevich A.B. Depression in general medical practice. - M.: Bereg, 2000. - P. 65-72. 2. Mosolov S.N., Smulevich A.B., Nuller Yu.L. and others. Use of the drug Paxil (Paroxetine) in the treatment of panic disorder (results of a Russian multicenter study). - M., 2003. 3. Praag HM van. Nosologomania: a disorder of psychiatry // World J. Biol. Psychiatry. - 2000. - 1. - 151-8. 4. Regier DA et al. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders // Br. J. Psychiatry. - 173 (Suppl. 34). - 24-8.
Antidepressant withdrawal symptoms
Withdrawal symptoms most often occur within three days of stopping antidepressants. Usually the symptoms are not severe and disappear within two weeks.
Symptoms of antidepressant withdrawal include:
- anxiety,
- depression and mood swings,
- dizziness,
- problems with balance,
- electric shock sensation
- headache,
- fatigue,
- flu symptoms (chills, muscle pain),
- loss of coordination
- muscle twitching and spasms,
- nausea,
- sleep disorders (nightmares, vivid dreams, insomnia),
- vomit.
Having antidepressant withdrawal symptoms does not mean that you are dependent on the drug.
Addiction represents harmful long-term chemical changes in the brain. It is characterized by intense cravings, an inability to control the use of a substance, and negative consequences of using that substance.
In rare cases, stopping an antidepressant can cause mania. Antidepressants, namely monoamine oxidase inhibitors, can lead to confusion and psychotic symptoms. If you are thinking about stopping an antidepressant, you should see a mental health professional to help prevent or minimize unwanted symptoms and discuss the risks and benefits of stopping treatment.
In many cases, stopping most antidepressants involves gradually reducing the dose of the antidepressant over several weeks or more under your doctor's supervision. This technique allows the brain to adjust to chemical changes and may prevent drug withdrawal symptoms. Do not try to reduce the dose of the drug or stop it on your own.
In some cases, your doctor may prescribe additional medications to ease withdrawal symptoms such as nausea and insomnia. Your doctor may also recommend switching from short-acting to long-acting antidepressants to ease withdrawal symptoms.
It can be difficult to distinguish withdrawal symptoms from depression symptoms after stopping an antidepressant. If you have any questions, you can make an appointment for a consultation by calling the Yusupov Hospital phone number. A psychiatrist provides assistance to patients around the clock.
Psychiatry Psychiatry and psychopharmacotherapy named after. P.B. Gannushkina No. 04 2004
N
Despite significant advances in the treatment of depressive conditions and a huge variety of drugs with antidepressant effects (there are more than 100 of them in the world), this problem remains one of the most pressing in modern psychopharmacotherapy. The emergence of modern drugs in the arsenal of psychotropic drugs, united by the concept of “new generation antidepressants,” opens up a broad prospect for a differentiated approach to the treatment of depression and poses the task of adequate drug selection to clinicians depending on the characteristics of the clinical picture and the severity of the patient’s condition. The first step towards solving this problem is to determine the spectrum of clinical action of new antidepressants (G.Ya. Avrutsky et al., 1988; S.N. Mosolov, 1995). One of the representatives of the new generation of antidepressants is the drug Paxil (paroxetine). It has a complex bicyclic structure, different from the structure of other known thymoanaleptics, and belongs to the most studied group of modern antidepressants to date - selective serotonin reuptake inhibitors (SSRIs). They are distinguished from tricyclic antidepressants (TCAs) by the almost complete absence of side effects with comparable effectiveness. The mechanism of action of paroxetine is based on its ability to selectively block the reuptake of serotonin (5HT) by the presynaptic membrane, which is associated with an increase in the free content of this neurotransmitter in the synaptic cleft and an increase in the serotonergic effect in the central nervous system, responsible for the development of the thymoanaleptic effect. The effect on muscarinic a- and b-adrenergic receptors is insignificant, which determines the extremely weak severity of anticholinergic, cardiovascular and sedative side effects. Among the serotonergic drugs, paroxetine is the most potent and one of the most specific serotonin reuptake blockers (DRThomas et al., 1987; IFTulloch et al., 1992). It is believed that the power of the serotonergic action is the main factor determining the severity of the thymoanaleptic effect, and selectivity determines tolerance. The results of numerous clinical studies show that paroxetine is highly effective in the treatment of major depression and associated symptoms, such as anxiety, ideational and psychomotor retardation, sleep disorders (J. L. Laghorn et al., 1992; A. Kiev, 1992; K. Rickels et al. , 1992; WT Smith et al., 1992). In randomized, double-blind studies, including more than 2.5 thousand patients with severe depression, it was found that paroxetine is not inferior in its effectiveness to imipramine (GCDunbar et al., 1991), amitriptyline (ALLaursen et al., 1985; H. Kuhs et al., 1989; H. Kuhs et al., 1990; A. Bignammi et al., 1992; P. Stott et al., 1993) and clomipramine (C. Link et al., 1992). According to the results of all these studies, the tolerability of paroxetine turned out to be better in relation to comparator drugs. In addition, paroxetine did not differ from amitriptyline in terms of its effect on anxiety symptoms (P. Stott et al., 1993), and compared to imipramine, it had an earlier effect (GCDunbar et al., 1991). The purpose of this work was to study the characteristics of the thymoanaleptic effect and tolerability of paroxetine. The study was conducted during the period 1998–1999. in four clinical centers in Russia: Moscow Research Institute of Psychiatry of the Ministry of Health of the Russian Federation, Scientific Center for Mental Health of the Russian Academy of Medical Sciences, St. Petersburg Psychoneurological Institute named after. V.M. Bekhterev, Chelyabinsk State Medical Academy (Department of Psychiatry). Statistical analysis of the results was carried out by the research organization InnPharm. The study was open, non-comparative. The duration of the study was 8 weeks (week 1 was the “wash out” period for patients taking psychotropic medications at the start of the study, and 7 weeks of active therapy). The study included patients aged 18 to 65 years with an ICD-10 diagnosis of recurrent depressive disorder (F32.1), moderate or severe depressive episode (F33.1 or F33.2), bipolar affective disorder (F31), episode of moderate or severe depression (F31.3; F31.4). The minimum score when assessed on the 17-point Hamilton scale (HHS) was at least 17, and on the Global Clinical Impression Scale (GCSI) - at least 3 points. The study included patients who gave written informed consent to participate in it. Exclusion criteria were: the period of pregnancy and lactation in women or the possibility of pregnancy during the study period; high suicide risk for outpatients; any clinically significant uncompensated diseases of the kidneys, liver, cardiovascular, respiratory system, cerebrovascular disorders or other serious progressive somatic diseases; organic diseases of the central nervous system, angle-closure glaucoma, prostatic hypertrophy, any clinically significant pathology detected during a physical examination and/or clinically significant abnormalities in laboratory parameters identified during the screening period; any of the listed treatment methods at the specified time intervals before the start of the study: MAO inhibitors (including reversible) - 2 weeks; electroconvulsive therapy – 3 months; depot forms of antipsychotics – 4 weeks, history of hypersensitivity to paroxetine, simultaneous prescription of type 1 antiarrhythmic drugs. For patients who were taking any psychotropic medications before the start of the study, a 7-day “wash out” period was carried out. In the first 2 weeks of the study, including the “withdrawal” period, the second on the 28th day of therapy), 4 were excluded due to adverse events (three on the 7th day of therapy, one on the 21st day of therapy).
The average duration of the current episode of depression was 3.6±2.02 months. Before the start of the study, drug treatment of the current depressive episode was carried out in 32 (49.2%) patients, non-drug treatment – in 2 (3.1%). The initial group average of the total score on the GS was 25.5±2.5. According to the GCS, the group average severity of depressive disorder was 2.9±0.8 points.
In the majority of patients (45–70.3%), the severity of the disease was assessed as “moderately severe mental disorders.” Paroxetine was prescribed in a single daily dose of 20 mg in the morning. If there was insufficient effectiveness, the dose was increased to 60 mg/day. The dose was increased gradually - by 10 mg at intervals of one week. The group average daily dose of paroxetine was 32.3±2.6 mg. When analyzing the data, the following results were obtained. The overall effectiveness of therapy at the end of the study according to SH (the percentage of patients with a reduction in the total score of at least 50%), calculated by the LOCF method, was 84.4% (54 out of 64 patients). When analyzed using the OS method, including only cases who completed the study, the effectiveness was 88.1% (52 of 59 patients) (Fig. 1). With both methods of data processing, already from the 7th day of therapy, the number of responders reached the level of statistical significance (p<0.001).
According to SHGKV, a positive effect (pronounced and significant improvement) was observed in 89.9% of patients (Fig. 2). At the same time, the severity of depressive symptoms before the start of therapy did not play a decisive role in its subsequent effectiveness. Thus, out of 45 patients with an initial assessment of “moderately severe mental disorders”, 17 were considered healthy at the final visit, in 13 the severity of the disease was assessed as “borderline state”, in 8 – as “mild mental disorders”, in 6 – no change in the severity of the disease was observed, i.e. “moderate severity of the disease” remained, in 1 there was a deterioration in the condition with an assessment of “severe impairment”. Of the 13 patients with an initial GCS assessment of “severe mental disorders,” at the final visit, 5 were considered healthy, 7 had a “borderline state” of severity, and 1 had “mild mental disorders.” Of the 3 patients with a “severe mental disorder” assessment, 1 had “mild mental disorders” at the final visit, and 2 had a “borderline” state.
“Mild mental disorders” were detected in 3 patients, of whom at the final visit 2 were considered healthy and in 1 the condition was assessed as “borderline”. The effect of therapy occurred quite quickly. The value of the total SH score for the group as a whole decreased by 19.9% already on the 7th day of treatment (the changes are statistically significant, p<0.001) (Fig. 3). Subsequently, there was a consistent decrease in the severity of depressive symptoms. By the end of the study, the total SH score was 7.5±2.6 points, i.e. in comparison with the initial level, its value decreased by 18.0±2.9 points (p<0.001), or by 69.9±5.6% (p<0.001). The rate of reduction of depressive symptoms according to the ShG is shown in Fig. 1. Already by the 28th day, the percentage of patients with a 50% reduction in the total GS score was 54.7. It should be noted that an “increase” in the number of patients with a positive effect of therapy was observed up to the 6th week of treatment.
As can be seen from Fig. 1, on the 35th day of treatment it was registered in 76.6% of patients, and over the next week the number of responders increased by another 7.8%, and subsequently this figure remained unchanged until the end of the study. The assessment of the speed of onset of the effect according to the ShGKV generally did not differ from that of the ShG. A change in the severity of the disease was observed already on the 3rd day of therapy (p<0.001) and exceeded the 50% level on the 28th day (Fig. 4). Throughout the study, the change in this indicator was fairly uniform, and a decrease in its value occurred until the end of observation (49th day - by 76%). This reflects the observed clinically smooth progressive reduction of depressive symptoms during treatment with paroxetine.
The number of patients with a positive effect of therapy (significant or pronounced improvement), as assessed by the ShG, exceeded 50% by the 28th day of treatment and increased until the 49th day (Fig. 5). These data confirm the validity of recommendations common to all SSRIs on the advisability of assessing the effect of therapy no earlier than 5–6 weeks from the moment of its initiation.
The main indicators that determine the spectrum of psychotropic action of the antidepressant (low mood, mental anxiety and lethargy) were reduced quite evenly (Fig. 6), although statistical significance in the reduction of lethargy was observed somewhat later (7th day of therapy) compared to symptoms of mental anxiety and decreased mood (3rd day of therapy). Clinically, already from the first week of treatment, the majority of patients noted an improvement in their well-being. First of all, the severity of anxiety decreased, and it became possible to stop taking anxiolytics prescribed during the “wash out” period. Patients became more contactable, more active in conversation, and noted that the interest in the usual range of activities and hobbies that had been lost during the period of depression was beginning to be restored. Analysis of the study results shows that paroxetine is quite effective in eliminating sleep disorders associated with depression. Clinically, this manifests itself as a gradual increase in the duration and then an improvement in the quality of sleep in the absence of a truly hypnotic effect.
According to TSTG, sleep indicators improve already from the 3rd day of therapy. By the end of the 1st week, the values of the indicators “difficulty falling asleep” and “early awakenings” change statistically significantly, and by the 21st day of treatment – “insomnia in the middle of the night” (Fig. 7). Paroxetine was generally well tolerated by patients. No clinically significant changes in laboratory parameters were observed. During treatment with the study drug, 65 cases of AEs were recorded in 25 of 65 patients (38.5%), but the association of the development of AEs with paroxetine was assessed as “probable” only in 14 (21.5%) cases. Assessment of the intensity of AEs showed that 33/65 (50.8%) cases of AEs were of mild intensity, 28/65 (43.1%) were of moderate intensity and 1/65 (6.2%) were of severe intensity. In most cases [40/65 (61.5%) AEs], no measures were taken regarding the study drug, in 18/65 (6.2%) cases the drug dose was reduced, in 4/64 (6.2% ) – increased and in 3/65 (4.6%) cases the drug was discontinued. Due to the development of AEs, 4 patients were excluded from the study. Of these, one patient with a burdened allergic history and drug intolerance to previous fluoxetine therapy experienced nausea and vomiting. The second one developed meningeal symptoms in the 2nd week of therapy, and was subsequently diagnosed with serous meningitis, and therefore the patient received specific therapy. The third patient had an allergic skin reaction similar to that previously observed when taking amitriptyline. In the fourth patient, the reason for discontinuation of therapy was nausea, which developed in the 1st week of therapy when taking 20 mg of paroxetine. All AEs that occurred during the study period and occurred with a frequency of more than 1 case are presented in Table. 1.
As can be seen from table. 1, the largest share among AEs is occupied by insomnia, anxiety, drowsiness, nausea and tremor. Analysis of AE outcomes showed that in the vast majority of cases (80%) AEs were transient. They developed in the first week of therapy when using a dose of 20 mg/day (Table 2) and subsequently decreased as therapy continued. With an increase in the dose of the drug in most patients, there was no increase in the frequency of AEs.
In general, this may indicate that either adaptation to paroxetine occurs in the first week of therapy, or some of the registered AEs (such as sleep disturbances, anxiety, somatovegetative disorders) were due to the instability of the patients’ mental state. Thus, summarizing the results of the study, we can conclude that paroxetine has a distinct thymoanaleptic, anxiolytic and activating effect, i.e. is an antidepressant with balanced action. Particularly important is the possibility of its effective use in the treatment of moderate and severe depression, including in hospital settings, shown by the study. Good tolerability of the drug, the transient nature of adverse events, their insignificant severity, as well as high compliance are its important advantages in the treatment of patients.
Currently, antidepressants are used for pharmacotherapy of generalized anxiety disorder (GAD) [12], which allow achieving remission in 40-50% of patients. In a number of studies [1, 7, 13, 14, 19], preference when choosing antidepressants is given to selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs). Antidepressants have been proven to be effective against both ideational and somatic anxiety [2, 5, 6, 16-18]. It has been revealed that they have a stronger effect on ideation anxiety compared to tranquilizers [11, 15].
Almost all known SSRIs are currently used for GAD [8]. But a special profile of receptor interaction sets paroxetine apart from a number of serotonergic drugs [3]. Its effectiveness in GAD has been proven in a number of placebo-controlled studies [18, 19]. At the same time, the superiority of paroxetine over benzodiazepine tranquilizers in reducing the symptoms of ideation anxiety was established. However, a number of important aspects of the use of paroxetine in GAD remain poorly understood, especially given its clinical heterogeneity. So, Yu.E. Less [4] identified 4 clinical types of this disorder: affective, somatized, phobic and tonic. In patients with the affective type of GAD, the foreground in the clinical picture is the actual affect of anxiety (the so-called free-floating anxiety). With the somatized type of GAD, anxious experiences are accompanied by pronounced autonomic hyperactivity. The phobic type of GAD is characterized by a “struggle” with pronounced anxiety, which brings it closer to the phenomenon of obsession. With the tonic type, muscle tension, a feeling of stiffness, and the inability to relax with a minimum of autonomic disorders are especially pronounced. The clinical heterogeneity of GAD is also confirmed by the fact that in no more than half of the patients the condition is exhausted by the phenomena of generalized anxiety throughout the entire disease. In other cases, at one or another stage of the disease, other comorbid disorders may appear, primarily of the affective and anxiety spectrum (panic disorder, obsessive-phobic disorders, agoraphobia, simple phobia, depression, dysthymia). It remains unclear how the type of GAD and the listed comorbid disorders affect the effectiveness of treatment with paroxetine.
Currently, a drug from the paroxetine group has appeared on the domestic market under the trade name “adepress”. This drug is therapeutically equivalent to the original paroxetine and is available in the form of tablets containing 20 mg of the active substance.
The purpose of the study was to study the effectiveness of Adepress in various clinical variants of GAD, taking into account comorbid conditions.
Material and methods
The work was carried out in the Department of New Means and Methods of Therapy, Department of Borderline Psychiatry, State Scientific Center for Social and Forensic Psychiatry named after. V.P. Serbsky on the basis of the clinical department of the Moscow City Clinical Psychiatric Hospital No. 12.
The study was an open naturalistic study.
The criteria for inclusion of patients in the study were as follows: diagnosis according to ICD-10 - F41.1 - generalized anxiety disorder; the presence of GAD as the main disorder, including comorbid symptoms of the anxiety-phobic circle (agoraphobia, panic disorder, social phobia, obsessive-compulsive disorder); the number of points on the Hamilton Anxiety Scale (HAM-A) is at least 20 at the time of inclusion in the study; patient age ranges from 18 to 60 years; availability of informed consent to participate in the study.
Exclusion criteria were: schizophrenia, organic disease of the central nervous system, drug addiction, alcoholism; the number of points on the HAM-D scale is more than 15 at the time of inclusion in the study; pregnancy or lactation; clinically significant somatic diseases or laboratory abnormalities.
The study included 49 hospitalized and outpatient patients (13 men and 36 women).
The duration of the study was 42 days. The initial dose of Adepress was 10 mg/day, followed by a flexible dosing regimen in the range of 10-60 mg/day. Adepress was used as monotherapy. In case of severe anxiety, as well as insomnia, the use of small doses of benzodiazepine tranquilizers (alprozolam, lorazepam, phenazepam) was allowed in the first days of treatment.
The patients' condition was assessed using the Hamilton Anxiety Scale (HAM-A) before starting therapy and then over time - weekly. Reduction of symptoms to 7 points or less was assessed as the level of remission; 50% reduction in the sum of points - as a pronounced improvement (responders); reduction by 25-49% - as a slight improvement (partial responders); reduction of symptoms less than 25% - as no effect (non-responders).
Tolerability of the study drugs was assessed using the side effects scale (UKU).
The routine examination also included the somatic condition of the patients (body weight, blood pressure, heart rate, ECG, general and biochemical blood tests, general urinalysis).
Patients were examined before treatment (day 0) and then on days 7, 14, 21, 28, 35 and 42 of therapy.
4 patients dropped out during the 1st week of therapy. In 2 cases, the reason for exclusion from the study was the use of prohibited drugs, in one case it was a violation of the dosage regimen, and in another case it was withdrawal of consent. A common reason for discontinuation from the study was increased anxiety, with insomnia in one case.
Thus, 45 patients completed the study: 12 (26.7%) men and 33 (73.3%) women, whose average age was 36.2 ± 8.2 years. Of these, 31 (68.9%) people worked or studied, 14 (31.1%) did not work; 29 (64.4%) were married, 16 (37.6%) were single or divorced.
In accordance with the clinical types of GAD identified in previous studies, patients were distributed as follows: somatized type - 16 patients, affective type - 8, phobic - 9, tonic type - 11.
As follows from numerous publications [9, 10, 17, 18], the study of the anti-anxiety effect of antidepressants is carried out with an analysis of their impact on the cognitive (mental) and somatic (vegetative) components of the anxiety state. Since the study sample included patients with a predominance of somatic anxiety (21 patients) and a predominance of cognitive anxiety (24), the sample was divided into 2 corresponding groups. The average HAM-A score in group 1 (with a predominance of somatic anxiety) was 27.4 points, in group 2 (with a predominance of cognitive anxiety) - 28.3 ( p
>0.05), which indicated their comparability.
There were 19 (42.2%) cases of isolated GAD in the studied sample, while 26 (57.8%) cases of GAD occurring with comorbid mental disorders. It turned out that in group 1 (somatic anxiety), isolated GAD was more common (73.7%), and in group 2 (cognitive anxiety), comorbid GAD was more common (61.5%). Of the comorbid disorders in group 1, only panic attacks occurred - 10 (41.7%) cases. In group 2, the spectrum of comorbid disorders included dysthymia - 7 (33.3%) cases, obsessions - 8 (38.1%), panic attacks - 1 (4.8%).
Statistical processing of the results was carried out using the Student's test, qualitative indicators - using the χ2 method, comparative analysis of the dynamics of quantitative indicators - using the one-way analysis of variance method, comparative analysis of ordinal data - using the Mann-Whitney test.
The characteristics of mental disorders, including comorbid ones, in the study sample are presented in Table. 1.
Results and discussion
Positive dynamics in most cases were noted by the end of the 1st week of therapy; after 2 weeks of treatment, significant differences from the initial level were recorded in both groups (Fig. 1).
Figure 1. HAM-A indicators during Adepress therapy in groups 1 and 2 of patients. Differences compared to background p < 0.05;
* — significant differences between the two groups, established in χ2 (p<0.05). Further, the HAM-A score in both groups continued to decrease, reaching minimum values by the end of the study. At the same time, more pronounced dynamics were noted in group 1, and from the 3rd week, intergroup differences became significant ( p
<0.05).
The overall results of the effectiveness of therapy are given in table. 2.
The remission rate in both groups was approximately the same (29.2 and 28.6%), while the number of responders and non-responders differed significantly. In the 1st group of patients with somatic anxiety, by the end of the study there were approximately 2 times more responders (50% versus 28.6%), and in the group of patients with cognitive anxiety there were non-responders (42.8% versus 20.1%). Most cases of a positive reaction to Adepress therapy in group 1 occurred earlier than in group 2. As can be seen in Fig. 2, most of the responders were detected after 3 weeks of therapy, and after 4 weeks almost all remissions were formed.
Figure 2. Distribution of patients (%) in different groups according to therapeutic response over time.
The abscissa axis is weeks. In the cognitive anxiety group, the majority of respondents were also noted after 3 weeks of treatment, but a deeper reduction of symptoms, reaching the level of remission, in most cases occurred only by the end of the 6-week treatment period. It should also be noted that the best results in the group of patients with a predominance of somatic anxiety were achieved using a relatively lower dose of Adepress. The average dose at the end of the study in group 1 was 14.4 mg/day, in group 2 - 32.8 mg/day ( p
<0.05).
A comparative analysis of the therapeutic response in patients with isolated and comorbid GAD revealed the following results. In group 1, with isolated GAD, 42.8% of patients had remission, 50% were responders, and only 7.1% of patients were non-responders. Different ratios were found in this group for comorbid GAD: remissions - 10%, responders - 50%, non-responders - 40% of observations. In the group of cognitive anxiety, with isolated GAD, remissions were 80%, non-responders - 20%, with comorbid GAD, remissions were noted in 12.5% of patients, responders - 37.5%, half of the patients with comorbid GAD in this group turned out to be responders. Thus, the presence of comorbid symptoms adversely affected the results of therapy in both groups of patients.
When analyzing the results of therapy depending on the clinical type of GAD, the following differences were revealed (Table 3).
From the table Table 3 shows that the highest rates of effectiveness of Adepress were achieved in the affective type of GAD, where clinical improvement was recorded in all observations (remission - 37.5%, responders - 62.5%). In second place was the phobic type of GAD (36.4%), however, this type had the fewest responders (18.2%) and the highest frequency of no effect (45.4%). In the somatized type of GAD, 25% of remissions were observed, which, together with 43.7% of responders, totaled more than ⅔ of patients of this type with a positive response to Adepress therapy; non-responders accounted for 31.2% of cases. With the tonic type of GAD, 20% of remissions, 40% of responders and the same number of non-responders were recorded.
As for the tolerability of Adepress, during the study, certain side effects were identified in 27 patients; in 18 patients they were of a combined nature. All adverse events could be divided into 2 groups. Group 1 included so-called mental adverse events, manifested by increased anxiety and agitation (7 observations). They were recorded in the 1st week of therapy and appeared to be associated with the “hyperstimulation” phenomena described during therapy with serotonergic antidepressants. These phenomena, although pronounced, were quite short-term in nature and, as a rule, were stopped by the prescription of small doses of tranquilizers. The 2nd group of adverse events included somatic side effects from the gastrointestinal tract, represented by nausea and diarrhea (5 observations). These phenomena occurred in the first half of the study (until the 28th day), were subjectively moderately painful, but did not require drug correction and resolved within a few days on their own. Drowsiness during Adepress therapy was observed in 12 cases, occurring at the initial stage of therapy (1-2 weeks). The severity of daytime sleepiness was insignificant, and taking into account the characteristics of the mental state of the patients included in the study, these adverse events were perceived as partly positive, allowing them to reduce “internal tension.” Moderate headache (6 observations) occurred in the first days of therapy, hyperhidrosis (5) on days 14-21, sexual dysfunction (6) on weeks 4-6 of therapy. These side effects, described in detail in the literature, are associated with stimulation of serotonin receptors in the central and peripheral nervous system, which is characteristic of all serotonergic drugs, including Adepress.
It should also be noted that adverse events occurred more often in patients with the somatized type of GAD, which included all 4 patients who dropped out of the study, as well as 50% of all registered patients studied. On average, 1.4 adverse events occurred per patient with the somatized type, 1.0 with the affective type, 0.7 with the tonic type, and 0.4 with the phobic type.
The study showed the high effectiveness of Adepress in patients with GAD. It has been established that this drug is effective both in isolated and in GAD complicated by comorbid mental pathology. However, the results of Adepress therapy for isolated GAD are superior to the corresponding indicators in the presence of concomitant psychopathological symptoms in the structure of GAD. The results obtained are consistent with the literature that comorbid disorders in GAD complicate pharmacotherapy, requiring an increase in the drug dose and timing to achieve a therapeutic response. It should be noted that in cases of a positive response to therapy, dysthymia, obsessions, and phobias comorbid with generalized anxiety, as a rule, are reduced simultaneously with the symptoms of generalized anxiety.
The present study also showed that adepress is effective when both somatic and cognitive anxiety are dominant, however, with the predominance of somatic anxiety in the structure of GAD, the effect occurs faster - in the 3-4th week of therapy; with GAD with a predominance of cognitive anxiety, the reverse development of symptoms occurs only at 3-6 weeks of therapy.
Adepress is effective in treating patients with any clinical type of GAD, but patients with GAD of the affective and somatovegetative type are more sensitive to Adepress therapy.
The absence of significant side effects indicates that Adepress is well tolerated. In accordance with the standards for the use of modern SSRIs, the tolerability of Adepress is differentiated for different types of GAD: the frequency of adverse events decreases in the following order: somatized, affective, phobic, tonic type of GAD.
Thus, Adepress is not only effective in the treatment of various clinical variants of GAD, but is also well tolerated, which allows the drug to be considered as a first-choice treatment for this disorder.
Duration of taking antidepressants
According to numerous studies, the duration of taking antidepressants is at least 6 months after the patient begins to notice improvement.
Patients who stop taking medications for up to 8 months may experience a return of symptoms. For patients who have had one or more relapses of depression, the duration of taking antidepressants is about 24 months. And those patients who experience frequent relapses of depression require long-term treatment, which can last several years.
To correctly select the dose of the drug and determine the duration of treatment, the Yusupov Hospital employs psychiatrists of the highest category with a scientific degree. Each patient is given close attention. Patients who seek medical help at the clinic are constantly under the supervision of a specialist throughout the entire treatment. Psychiatrists can be consulted about rehabilitation after treatment, which makes it possible to reduce the incidence of recurrent symptoms of depression.
Independent and uncontrolled use of antidepressants entails the development of unwanted side effects, which in turn leads to early drug withdrawal and ineffective treatment.
Expert opinion
Author:
Elena Mikhailovna Bunina
Psychiatrist, doctor of the highest category
Statistics show that 5% of the population suffers from symptoms of depression. In most cases, psychiatrists decide to prescribe antidepressants. The drugs are included in the list of complex therapy used for depressive disorder.
Antidepressants work by restoring normal levels of chemicals responsible for regulating sadness and anxiety. Numerous clinical studies have proven that drugs of this pharmacological group are not addictive. However, one in five experience withdrawal symptoms. They are associated with abrupt cessation of taking prescribed medications. It is impossible to predict the development of withdrawal symptoms in advance.
Psychiatrists at the Yusupov Hospital select an individual treatment plan for each patient. It includes complex therapy using antidepressants that have undergone quality and safety control. The medications have proven effectiveness and are included in the list of world standards for the treatment of depression. Doctors do not recommend stopping the prescribed course on your own or abruptly. This can worsen the situation and lead to the development of unwanted symptoms.