Antienzyme drugs (proteolysis inhibitors) are a group of drugs that block the action of digestive pancreatic enzymes and are used to treat acute pancreatitis.
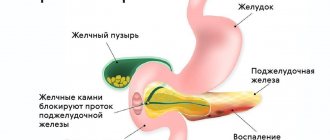
The pancreas is an organ of mixed secretion. Its endocrine part, which is represented by the islets of Langerhans, produces hormones directly into the blood that affect carbohydrate metabolism - insulin and glucagon. The exocrine part of the pancreas produces pancreatic juice, which is critically necessary for normal digestion, which is secreted through the duct into the lumen of the duodenum.
Pancreatic juice is a mixture of enzymes that can break down all the main components of food - proteins, fats and carbohydrates - into simple compounds that can be further absorbed in the small intestine.
Pancreatic enzymes (pancreatic enzymes) have extremely powerful “digestive” powers, so the pancreas synthesizes them in an initially inactive (zymogenic) form. Under normal conditions, the transition of pancreatic enzymes to an active form occurs only in the lumen of the duodenum, when interacting with food components.
In case of disruption of the outflow of pancreatic juice, for example, in acute diseases of the pancreas, enzymes are activated directly in the tissue of the gland itself. This, in turn, leads to so-called autolysis - “self-digestion” of the pancreas and enzymatic “melting” of surrounding tissues.
Autolysis of the pancreas is an acute condition that threatens human life. In the absence of adequate assistance measures, there is a high risk of death for the patient.
One of the basic groups of drugs that are used for the prevention and treatment of autolysis of the pancreas includes anti-enzyme drugs - proteolysis inhibitors.
Indications for use
Antienzyme drugs are used for diseases that are accompanied by a violation of the outflow of pancreatic juice from the pancreas: acute pancreatitis, exacerbations of chronic pancreatitis, severe injuries of the pancreas, pancreatic tumors, swelling of the walls of the duodenum with long-term active intake of alcohol.
Anti-enzyme drugs are also used to prevent severe bleeding and severe blood loss in case of operations on the heart, cardiac (coronary) vessels, lungs, etc.
Integrase inhibitors: mechanism of action
Integrase, along with reverse transcriptase and protease, is one of the key participants in HIV-1 replication. It catalyzes processes leading to the movement of DNA or its fragments within the genome or between genomes. Being part of the pre-integration complex, integrase recognizes long terminal repeats at the ends of the double strand of newly synthesized viral DNA, cleaves two or three bases from each end, and also participates in the transport of viral DNA into the nucleus of the target cell, where it determines the preferred site for the introduction of viral DNA into the chromosome and carries out its integration into the cellular genome [5].
Integrase inhibitors, by binding to its active site and blocking the strand transfer stage during the integration of retroviral DNA, prevent the formation of covalent bonds with the host DNA and the incorporation of HIV into the host genome [4].
Unlike reverse transcriptase and protease, integrase has no close analogues in the eukaryotic cell, therefore, drugs that selectively act on it should have minimal side effects [5].
The first protease inhibitor was raltegravir, approved by the US Food and Drug Administration in 2007 [6]. Today, along with raltegravir, 4 more drugs of this class are used: elvitegravir, dolutegravir, bictegravir and cabotegravir. There are two more molecules in development, one of which is likely to have a duration of action up to 4 times longer than raltegravir and may be classified as a second-generation integrase inhibitor.
pharmachologic effect
Antienzyme drugs block the most powerful pancreatic enzyme, trypsin. Trypsin breaks down proteins (proteins) through a biochemical process called proteolysis. That is why antienzyme drugs are also called proteolysis inhibitors. At the same time, the processes of self-digestion in the pancreas stop and toxins - autolysis products - stop being released into the blood.
Antienzyme drugs additionally have a hemostatic effect by blocking the action of other protease enzymes in the blood responsible for the breakdown of proteins. In particular, antienzyme drugs inhibit the activity of the enzyme fibrinolysin (plasmin), which is responsible for the destruction of fibrin threads that stabilize blood clots, thereby preventing bleeding from stopping.
In addition, antienzyme drugs have a nonspecific anti-inflammatory effect.
Protease inhibitors: mechanism of action
The introduction of protease inhibitors into clinical practice has become a cornerstone of HIV treatment. Their addition to the treatment regimen with two nucleoside reverse transcriptase inhibitors (NRTIs) made it possible to reduce the number of patients with a progressive course of the disease (before the development of AIDS or death) by half. In 90% of patients receiving the three-drug combination, the number of HIV RNA particles in the blood decreased from more than 20,000 per milliliter to less than 500 per milliliter after 24 weeks of therapy [1].
The mechanism of action of protease inhibitors is based on their ability to block one of the enzymes that serves as an important element of virus maturation in the life cycle of HIV. The HIV genome encodes a long polypeptide. After removal of the viral envelope and reverse transcription of the RNA genome, a polypeptide is formed containing all the viral gene products, including structural proteins and enzymes. HIV protease then breaks it down into its component viral proteins. When protease activity is suppressed, this process is blocked, which leads to impaired maturation of viral mRNA, resulting in the formation of viral particles that are unable to infect new cells [1].
The first protease inhibitors were peptidomimetic molecules, similar to and competitive with the precursor peptide. However, like most peptidomimetic proteins, early protease inhibitors had a significant pharmacokinetic disadvantage: they were poorly absorbed and rapidly eliminated when taken orally. The problem was solved by replacing the pyridine site in the structure of protease inhibitors with a thiazole, which is less electron-rich. Thanks to this, it was possible to increase the metabolic stability of the molecule and improve the solubility of the substance in water, as well as increase its effectiveness against the virus [1, 2].
The first protease inhibitor approved by the US Food and Drug Administration (FDA) was saquinavir, which received the green light just 97 days after its application was submitted. Over the next few months, two other protease inhibitors, ritonavir and indinavir, received marketing approval. Today, ten drugs in this class have regulatory approval: amprenavir, atazanavir, lopinovir, ritonavir, tipranavir, nelfinavir, indinavir, saquinavir, fosamprenavir and darunavir.
Basics of treatment with antienzyme drugs
Considering the peculiarities and difficulties in selecting an individual dose of antienzyme drugs for a particular patient, as well as a wide range of side effects and interactions with other drugs, proteolysis inhibitors are prescribed only by an experienced doctor in a hospital setting.
Aprotinin can cause a severe allergic reaction, including anaphylactic shock. It is strictly contraindicated to prescribe the drug to patients who have been administered aprotinin over the past year.
General properties of protease inhibitors
Drugs in this group reduce the concentration of viral RNA, increase the number of white blood cells responsible for the immune system's response to infection (CD-4 cells) and, ultimately, improve survival when included in therapy along with other classes of antiretroviral drugs.
In general, protease inhibitors have similar therapeutic effects. Their activity against HIV-1 and HIV-2 is assessed as high, and resistance to them develops slowly.
Pharmacokinetics
Protease inhibitors are extensively metabolized by isoenzymes of the cytochrome P450 system, in particular CYP3A4, which opens up the possibility of a number of drug-drug interactions. Agents that induce P450 enzyme activity, such as rifampicin or St. John's wort extract, accelerate the metabolism of protease inhibitors, reducing their plasma concentrations and therapeutic efficacy. Conversely, drugs that inhibit P450 enzymes, such as ketoconazole or cimetidine, increase plasma levels of protease inhibitors, which is associated with increased toxicity of the latter [2].
Currently, the most potent CYP3A4 inhibitor is ritonavir. It is specifically combined with other protease inhibitors that are CYP3A4 substrates to prolong their half-life [4].
Protease inhibitors are better absorbed when taken during or after meals [2, 3].
Indications
Drugs of this class are used to treat HIV infection, prevent mother-to-child transmission of infection, and post-exposure prophylaxis of the disease.
Contraindications
The simultaneous use of ritonavir with drugs whose elimination depends on the activity of CYP3A4 is contraindicated to avoid the toxicity of antiretroviral therapy.
Side effects
Adverse reactions with the use of protease inhibitors include [2, 3, 4]:
- gastrointestinal disorders, in particular, diarrhea with an unknown mechanism, which is complicated by the fact that it often becomes one of the complications of HIV;
- hyperlipidemia is a common side effect for all protease inhibitors, less common when taking atazanavir; Apparently, lipid metabolism disorders are associated with the redistribution of adipose tissue characteristic of HIV, which is aggravated by taking protease inhibitors;
- hyperglycemia, insulin resistance, which probably develop as a result of suppression of the glucose transporter by protease inhibitors, which leads to suppression of the activity of glucose uptake by cells; atazanavir is less likely to cause this side effect compared to other protease inhibitors;
- rash most often occurs when taking amprenavir.
A number of unique side effects are also known: for example, when taking atazanavir, hyperbilirubinemia may occur, which is not considered a sign of hepatotoxicity of the drug, and when using indinavir, crystalluria and nephrolithiasis occur, which is associated with the poor solubility of the drug in water. Therefore, patients receiving indinavir are advised to increase their fluid intake during treatment.
special instructions
The use of ritonavir as a single protease inhibitor is limited due to its significant digestive side effects at therapeutic dosages. Today the drug is used in low doses to increase the activity of other protease inhibitors. One of the most common combination drugs used to treat HIV today is lopinavir + ritonavir. It should be noted that ritonavir capsules and solutions contain ethyl alcohol, so patients receiving the drug should avoid taking drugs that cause a disulfiram reaction, in particular disulfiram and metronidazole [4].
General properties of protease inhibitors
The mechanisms of resistance to integrase inhibitors differ from those to other classes of antiretroviral drugs. If the basis of the resistance of most non-nucleoside reverse transcriptase inhibitors and protease inhibitors is a change in the configuration of the active center of the enzyme and the subsequent blocking of the addition of the drug molecule, then in the case of integrase inhibitors, in all likelihood, other phenomena occur, and they may differ for different mutations [7]. Raltegravir is considered to have a low genetic barrier to the development of resistance because a single point mutation in HIV can alter the ability of integrase inhibition. Cross-resistance between integrase inhibitors may occur [4].
Pharmacokinetics
The main route of elimination of raltegravir is through the liver, however, dose adjustment of the drug is not required in patients with mild to moderate hepatic impairment. Renal excretion plays a minor role in the metabolism of protease inhibitors, so the dose should not be adjusted in case of renal failure.
Raltegravir is not a substrate for cytochrome CYP450 enzymes, does not inhibit or induce them, which is associated with a low frequency of drug interactions.
Indications
Protease inhibitors are indicated for HIV-1 infection in patients who have developed resistance to other classes of antiretroviral drugs.
Contraindications
They have no therapeutic value due to the high safety profile of the drugs.
Side effects
Because antiretroviral drugs are usually prescribed in combination regimens, it is difficult to identify adverse reactions associated with a specific class or drug. In general, protease inhibitors are well tolerated. In controlled studies, side effects with raltegravir were comparable to those with placebo. Raltegravir is considered one of the safest drugs for the treatment of HIV infection due to the minimal risk of adverse events, both in patients who have not previously received antiretroviral therapy and who have already had experience in treatment with drugs of other classes [4, 8].