Buy Buserelin-depot lyophilisate for the preparation of suspension intramuscularly 3.75 mg in pharmacies
Buserelin-depot Buy Buserelin-depot in pharmacies DOSAGE FORMS lyophilisate for preparing a suspension for intramuscular injection. entered prolong. active 3.75 mg lyophilized powder for the preparation of intramuscular solution 3.75 mg
MANUFACTURERS Deco Company (Russia) Pharm-Sintez (Russia)
GROUP Antiandrogens
COMPOSITION Active ingredient: buserelin acetate 3.75 mg in the form of a free peptide.
INTERNATIONAL NON-PROPENTED NAME Buserelin
SYNONYMS Buserelin, Buserelin FSintez, Buserelin-long FS, Buserelin acetate solution
PHARMACOLOGICAL ACTION Pharmacodynamics. A synthetic analogue of natural gonadotropin-releasing hormone (GnRH). Buserelin competitively binds to the receptors of the cells of the anterior pituitary gland, causing a short-term increase in the level of sex hormones in the blood plasma. Further use of therapeutic doses of the drug leads (on average after 12-14 days) to a complete blockade of the gonadotropic function of the pituitary gland, thus inhibiting the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). As a result, there is a suppression of the synthesis of sex hormones in the gonads, which is manifested by a decrease in the concentration of estradiol in the blood plasma to post-menopausal values in women and a decrease in testosterone content to a post-castration level in men. The concentration of testosterone with continuous treatment for 2-3 weeks decreases to the level characteristic of the orchiectomy state, i.e. pharmacological castration is caused. Pharmacokinetics. Bioavailability is high. The maximum plasma concentration is reached approximately 2-3 hours after intramuscular administration and remains at a level sufficient to inhibit the synthesis of gonadotropins by the pituitary gland for at least 4 weeks.
INDICATIONS FOR USE Hormone -dependent prostate cancer, breast cancer, endometriosis (pre- and postoperative periods), uterine fibroids, endometrial hyperplastic processes, infertility treatment (during an in vitro fertilization (IVF) program.
CONTRAINDICATIONS Pregnancy, breastfeeding, hypersensitivity to the components of the drug.
SIDE EFFECTS Allergic reactions: urticaria, skin hyperemia, rarely - angioedema. In women, typical adverse reactions are a manifestation of the achieved hypoestrogenic state - “pharmacological menopause”: From the central nervous system: frequent mood swings, sleep disturbances, depression, headache. From the endocrinological status: hot flashes, increased sweating, vaginal dryness, decreased libido, pain in the lower abdomen, demineralization of bones, rarely - menstrual-like bleeding (usually during the first weeks of treatment). In men, during the treatment of prostate cancer - during the first 2-3 weeks after the first injection, it can cause exacerbation and progression of the underlying disease, which is associated with stimulation of the synthesis of gonadotropins and, accordingly, testosterone, gynecomastia, a transient increase in the concentration of androgens in the blood (rarely - ossalgia , urinary retention, renal edema, muscle weakness in the lower extremities, lymphostasis). Other: in isolated cases (the cause-and-effect relationship has not been clearly established) - pulmonary embolism, dyspeptic disorders.
INTERACTION The simultaneous use of Buserelin with drugs containing sex hormones (for example, in the mode of ovulation induction) may contribute to the occurrence of ovarian hyperstimulation syndrome. With simultaneous use, Buserelin may reduce the effectiveness of hypoglycemic agents.
DOSAGE AND ADMINISTRATION For hormone-dependent prostate cancer: 3.75 mg intramuscularly (IM) every 4 weeks; In the treatment of endometriosis, endometrial hyperplastic processes: 3.75 mg IM once every 4 weeks. Treatment begins in the first five days of the menstrual cycle. Duration of treatment - 4-6 months; For the treatment of uterine fibroids: 3.75 mg IM once every 4 weeks. Treatment begins in the first five days of the menstrual cycle. Duration of treatment - 3 months before surgery, in other cases - 6 months; When treating infertility using in vitro fertilization (IVF): 3.75 mg IM once on the 2nd day of the menstrual cycle.
OVERDOSE Currently, no cases of overdose with Buserelin have been reported.
SPECIAL INSTRUCTIONS For women. Patients with any form of depression during treatment with the drug should be under close medical supervision. Ovulation induction should be performed under strict medical supervision. In the initial stage of treatment with the drug, the development of ovarian cysts is possible. Before starting treatment with the drug, it is recommended to exclude pregnancy and stop taking hormonal contraceptives, however, during the first two months of using the drug, it is necessary to use other (non-hormonal) methods of contraception. In men. In order to effectively prevent possible side effects in the first phase of the drug’s action, it is necessary to use antiandrogens two weeks before the first injection of buserelin depot and for two weeks after the first injection. Influence on the ability to drive a car and other mechanisms. Caution should be exercised when prescribing the drug to patients engaged in potentially hazardous activities that require increased attention and speed of mental and motor reactions.
STORAGE CONDITIONS Store in a place protected from light and out of reach of children, at a temperature of 8 to 20
Results and discussion
List of efficiency parameters
The protocol provided for the following performance criteria:
1. Studies of hormone levels in the blood:
— decrease in LH level to 7.4±7.3 mIU/ml;
— decrease in FSH level to 7.0±5.6 mIU/ml;
- decrease in estradiol level <110 pmol/l.
2. Instrumental research:
- transvaginal ultrasound examination of the uterus and appendages - reducing the thickness of the median uterine echo to 4-6 mm. Changes in the pathological structures of the myometrium with varying degrees of adenomyosis during treatment;
- aspiration biopsy of the endometrium - changes in pathological structures in the endometrium or endometrial atrophy.
3. Assessment of health using the “Patient Questionnaire” - assessment of complaints, general condition, emotional state, physical condition, menopausal symptoms.
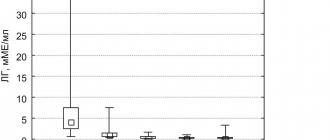
Dynamics of LH levels
. At the screening visit, as well as at visits 2–5, patients' blood was tested to determine LH levels (Fig. 1).
Rice.
1. Dynamics of LH levels during the use of the drug “Buserelin-long FS”. Achieving the target LH level <7.4±7.3 mIU/ml was achieved in 100% of patients by visit 2 after starting therapy with Buserelin-long FS.
By visit 5, there was a decrease in LH levels in all patients by an average of more than 6 times compared to the visit during the screening period.
Thus, 50 (100%) of 50 patients met the effectiveness criterion “Decrease in LH levels to 7.4±7.3 mIU/ml.”
Dynamics of FSH levels
. At the screening visit, as well as at visits 2–5, patients' blood was tested to determine FSH levels.
When analyzing the level of FSH during therapy with the drug "Buserelin-long FS" (Fig. 2), a statistically significant decrease in the level of this hormone was revealed at visits 2, 3, 4 and 5.
Rice. 2. Dynamics of FSH levels during the use of the drug “Buserelin-long FS”.
Thus, the patient population ( n
=50) generally satisfied the criterion “Decrease in FSH level to 7.0±5.6 mIU/ml”: the average FSH level in patients at visit 5 was 4.54±2.5 mIU/ml. A decrease in FSH levels by visit 5 was observed in 33 (66%) of 50 patients. Since, based on the literature, therapy with GnRH analogues does not always lead to a decrease in FSH levels in patients with endometriosis, other parameters of effectiveness should be taken into account when presenting a conclusion about the effectiveness of the drug.
Dynamics of estradiol levels
. At the screening visit, as well as at visits 2–5, patients' blood was tested to determine estradiol levels.
Already by visit 2, a statistically significant decrease in estradiol levels was registered, which persisted throughout the entire course of therapy (Fig. 3).
Rice. 3. Dynamics of estradiol levels during the use of the drug “Buserelin-long FS”.
Of the 50 patients, 32 (64%) achieved target estradiol levels of ≤110 pmol/L. In a study by U. Cirkel et al. [12] the level of estradiol in patients with endometriosis treated with buserelin decreased to an average of 164±32 pmol/l after 3 months of treatment, and in a study by W. Dmowski et al. [13] - up to 132±18 pmol/l, which is slightly higher than the 110 pmol/l value chosen as the end point in this study.
In 4 patients, a significant increase in estradiol levels was detected by visit 5, with a significant decrease in its levels by visits 3-4. In 1 patient, there was no significant increase/decrease in estradiol levels by visit 5, with a significant decrease in its level by visits 3-4. The reason could be a deviation in the date of visit 5 or the so-called “rebound effect,” when a short-term significant increase in the concentration of sex hormones is observed shortly after stopping the use of GnRH agonists.
Thus, 32 (64%) of 50 patients met the effectiveness criterion “Decrease in estradiol levels ≤110 pmol/l.” In general, for 45 (90%) patients out of 50, treatment with Buserelin-long FS was effective in terms of reducing estradiol levels, based on a significant decrease in estradiol levels relative to the initial level.
M-echo parameters
. At the visit during the screening period, as well as at visits 2–5, a transvaginal ultrasound examination of the uterus and appendages was performed to identify changes in the pathological structures of the myometrium in various degrees of endometriosis during treatment.
During treatment with Buserelin-long FS, a statistically significant decrease in endometrial thickness was observed already by visit 2 (Fig. 4).
Rice. 4. Changes in endometrial thickness during therapy with Buserelin-long FS.
Thus, 49 (98%) of 50 patients met the effectiveness criterion “Reducing the thickness of the median uterine echo to 4-6 mm.”
Results of endometrial aspiration biopsy
. To identify changes in the endometrium or endometrial atrophy during treatment with Buserelin-long FS, an endometrial biopsy was performed in patients during the screening visit and at visit 5. Based on the results of aspiration biopsy, in 33 out of 50 patients at visit 5, it can be concluded that in the majority of patients - 29 (88%) out of 33 - endometrial atrophy or a decrease in the degree of endometrial hyperplasia was observed at visit 5 in relation to the visit during the screening period.
Patient survey data.
At each visit, including the screening visit, patients completed a questionnaire. The questionnaire consisted of several questions, some of which made it possible to assess changes in the subjective perception of pain symptoms, in the physical and emotional state of the patients’ own health (“1. How would you rate the state of your health?”, “2. How would you rate the state of your health now compared to 4 weeks ago?", "3.1. To what extent does your health condition limit you from performing strenuous physical activities (lifting and carrying heavy objects, running, climbing several flights of stairs, walking several kilometers)? ", "3.2. To what extent does your health condition limit you from performing moderate physical activity (climbing one flight of stairs, walking several hundred meters, vacuuming or mopping the floor yourself)?", "3.3. To what extent does your health condition limits you from performing light physical activities (walking 100 m, dusting, getting dressed)?”, “4. How would you rate your physical condition now compared to 4 weeks ago?”, “5. How would you rate your emotional state compared to what it was 4 weeks ago?”, “6. How much physical pain have you experienced over the past 4 weeks?"). Patients could choose only one answer to each question.