Relevance
In February 2022, the French National Medical Agency (ANSM) recommended temporarily stopping the use of docetaxel in patients with infiltrative non-metastatic breast cancer.
French pharmacovigilance has previously reported potential serious adverse events associated with the use of Taxam. Taxams (docetaxel and paclitaxel) occupy an important place in the treatment of certain malignant neoplasms, for which there is sometimes no alternative treatment method. This class of drugs, like many others, have severe side effects.
On July 5, 2022, a safety report on the drug was presented on the ANSM website.
Basic questions for patients regarding chemotherapy
E.B. SHAKHNOVICH, oncologist, Federal State Health Institution Central Medical Unit No. 119, FMBA of Russia Medical Unit No. 4
Despite all the advances in medicine, the diagnosis of malignant oncological disease, in common non-medical vocabulary - cancer, remains the most psychologically unacceptable diagnosis, causing a feeling of fear and hopelessness in the patient.
Treatment of oncological pathology includes a number of techniques, such as surgical treatment, radiation therapy, antitumor treatment and many others. As a rule, antitumor treatment in non-medical vocabulary is called chemotherapy, although this concept includes a number of different drugs, such as chemotherapy itself (the use of cytostatic drugs), hormone therapy, immunotherapy, targeted therapy, etc. Drugs of these groups can be used both in combination with radiation therapy, surgical treatment, and in combination with each other, which is called combined or complex treatment.
In this article we will try to answer the main questions that arise in almost all patients who are prescribed chemotherapy treatment.
How chemotherapy drugs confuse cells
The main goal of antitumor therapy is to stop the growth of the tumor, reduce its size, reduce the clinical manifestations of the disease, and in the most favorable case, lead to its complete destruction. Each type of tumor has its own set and combination of drugs, and each patient has its own individual sensitivity and development of complications during chemotherapy, so you should never rely on other patients receiving antitumor treatment.
The mechanism of cytostatic drugs is based on the fact that these substances are released by rapidly dividing body cells (tumor cells), are integrated into the life processes of tumor cells, most often during the division process, and lead to the death of malignant cells. As a rule, several cytotoxic drugs are prescribed to have different effects on the functioning of the tumor cell, which is called a treatment regimen. Each drug has its own mechanism of action and, as a result, different types of toxicity.
In the process of creating and studying treatment regimens, drugs with different mechanisms of action and toxicity are selected, i.e. Cross (same) toxicity is eliminated whenever possible, which increases the tolerability of the treatment regimen and efficiency.
A feature of chemotherapy treatment is its duration and frequency, depending on the mechanism of action of the drugs. During the break between courses, the patient is exposed to the drug; repeated administration serves to create an optimal concentration of the drug in the blood in order to maximize the effect on the tumor. Doses of chemotherapy drugs are strictly individual for each patient and are calculated taking into account the patient’s height and weight (body surface area).
But in the human body, in addition to rapidly dividing tumor cells, there are also normal cells with rapid division according to their function. These include cells of hair follicles, bone marrow (hematopoietic precursor cells), intestinal epithelium, reproductive cells, etc. Chemotherapy drugs “confuse” these cells with the cells of a malignant tumor, which leads to complications: hair loss (alopecia), decreased levels of leukocytes (leukopenia, neutropenia), nausea and vomiting, loose stools (diarrhea), stomatitis, etc.
The most difficult psychological factor
Alopecia (hair loss) is not the most important phenomenon for the patient’s health, but it raises the first question about complications during chemotherapy, because is a psychologically difficult factor, especially for young women. Not all chemotherapy drugs cause hair loss. But if the patient undergoes preventive treatment after removal of the mammary gland for cancer with the use of Doxorubicin, Adriamycin, total hair loss is expected.
As a rule, maximum hair loss begins 10-14 days after chemotherapy is administered. In this case, in order to maintain personal hygiene, it is recommended to remove hair (shave your head) at the first signs of hair loss. Alopecia is reversible, hair restoration begins after the end of chemotherapy in approximately 2-3 weeks. Hair growth is very fast and according to our observations, the growth density increases, color changes and the appearance of curly hair growth are possible.
Not the most dangerous, but a common manifestation
Nausea and vomiting are not the most dangerous, but the most common and most painful manifestation of the toxic effect of chemotherapy drugs. There are acute nausea and vomiting, delayed and waiting vomiting.
Acute nausea and vomiting develops within 24 hours of chemotherapy administration. Delayed nausea and vomiting occurs more than 24 hours after treatment and may last several days. Anticipatory vomiting usually occurs before a second course of chemotherapy during long-term chemotherapy treatment and is psychogenic in nature. Vomiting of anticipation can be caused by the very thought of the upcoming chemotherapy the day before, the appearance of the hospital, the treatment room, specific smells, etc.
The main mechanism for the occurrence of acute vomiting is the activation of 5-HT3 receptors, which are located in the brain, neurons of the gastrointestinal tract (GIT), and the vagus nerve under the influence of serotonin produced in the cells of the upper intestine. In order to relieve this complication, pharmacology offers a sufficient range of drugs both in the form of solutions and tablet forms and suppositories. In the practice of Russian doctors, the most common drugs are Zofran (Latran, Emiset) in the form of tablets, injections or suppositories, Navobane in the form of capsules and solutions, Kytril in the form of tablets and solutions (drugs are ranked according to their effectiveness).
In order to increase the antiemetic effect of these drugs, glucocorticoid hormones are additionally used, most often dexamethasone, which, in addition to its antiemetic (antiemetic) effect, has antiallergic functions during chemotherapy. The choice of antiemetic therapy depends on the type of chemotherapy drugs, which are divided into highly emetogenic, moderately emetogenic and low emetogenic.
The mechanism of cytostatic drugs is based on the fact that these substances are released by rapidly dividing body cells (tumor cells), are integrated into the life processes of tumor cells, most often during the division process, and lead to the death of malignant cells. As a rule, several cytotoxic drugs are prescribed to have different effects on the functioning of the tumor cell, which is called a treatment regimen. Each drug has its own mechanism of action and, as a result, different types of toxicity.
Psychogenic nausea and vomiting
Initially, the prescription of anti-emetic therapy is carried out solely on the basis of the doctor’s experience; then, when an individual reaction is established, anti-emetic therapy can be changed towards intensification if the effect is insufficient. The time of administration and method of administration of the drug are determined by the instructions for use. The effect of antiemetic drugs lasts on average 8-12 hours, the effectiveness ceases 48 hours after the administration of chemotherapy and is meaningless, therefore, in order to prevent and relieve delayed vomiting, we recommend the use of cerucal, and in case of severe nausea or vomiting, the administration of dexamethasone.
All drugs in the 5-HT3 antagonist group act on intestinal activity and can cause constipation and, less commonly, diarrhea. In order to reduce toxic reactions of antiemetics, it is necessary to strictly observe the intervals between doses and the maximum permitted doses. Antiemetics do not prevent preliminary nausea and vomiting, because it is psychogenic in nature, the main drugs used for preliminary nausea or vomiting are sedatives (sedatives), selected strictly individually. The main complication of vomiting is dehydration, which can lead to heart failure due to electrolyte imbalance and venous thrombosis. If this complication occurs, it is recommended to drink plenty of mineral salts. If severe vomiting occurs more than 4-5 times a day for more than 2 days, you should consult a doctor to restore electrolyte balance by intravenous administration of electrolytes.
There is an opinion among patients
The nutrition of patients during chemotherapy treatment does not require adherence to any diet and remains typical for the patient’s daily routine nutrition. However, it is necessary to follow the diet recommended for concomitant diseases, such as diabetes, stomach ulcers, cholecystitis, etc.
There is an opinion among patients that consuming foods containing glucose promotes tumor growth or that consuming milk and fermented milk products contributes to the spread of breast cancer. But these statements are not true.
When administering chemotherapy, severe pain and a burning sensation may occur at the injection site or along the vein, which is due to the adverse effects of chemotherapy on the vascular endothelium. If these sensations occur, you should additionally “rinse” the vessel with saline solution after completing chemotherapy.
Repeated administration of chemotherapy can cause the development of phlebitis and phlebosclerosis of the veins of the limb. In order to prevent these complications, it is necessary to comply with the rules for diluting drugs and the duration of their administration according to the instructions. As an additional treatment, it is necessary to use alcohol or semi-alcohol compresses on the injection area, ointments containing anticoagulants.
In case of insufficient development of the superficial peripheral veins of the upper extremities, long-term infusions of 5-fluorouracil, and expected long-term repeated chemotherapy, it is possible to install an intravenous port, which allows treatment without the use of peripheral veins of the extremities.
Injection of chemotherapy into the arm from the surgical removal of the mammary gland should be avoided, because this can increase the symptoms of lymphostasis; taking blood from this arm is quite acceptable.
Is it possible to have children after chemotherapy?
When administering chemotherapy, an iatrogenic complication is possible: necrosis of the subcutaneous tissue when the drug gets under the skin. The occurrence of these complications depends on the practical skills of the medical worker administering the drug. The resulting necrosis is characterized by rapid spread through the subcutaneous tissue and a long process of tissue regeneration.
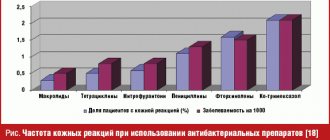
Allergic reactions during treatment can occur in 5-10% of patients when using various chemotherapy drugs; the most dangerous in this case are taxanes, which require specific pre- and post-medication with the inclusion of large doses of glucocorticoids. The occurrence of an allergic reaction is possible at any stage of treatment, regardless of the number of previously administered similar courses of chemotherapy.
Quite often, patients are concerned about the effect of chemotherapy on sexual function. Potency may decrease during chemotherapy, but after it is completed, erectile function is fully restored.
Chemotherapy has a significant impact on the growth and development of the egg, which can be accompanied by menstrual irregularities up to aminorrhea. Women over 40 years of age may experience menopause during chemotherapy, which in some cases requires consultation with a gynecologist.
The possibility of having children during chemotherapy is excluded for both men and women. Germ cells are highly sensitive to chemotherapy, which can lead to the development of genetic defects and the birth of an underdeveloped child. Abroad, before chemotherapy, women of reproductive age undergo a preliminary pregnancy test and sign a special document on the prevention of complications during pregnancy during chemotherapy.
It is more difficult to answer the patient’s question about the possibility of having children after treatment. There remains a risk of damage to the genetic apparatus of the egg and the development of genetic defects in the child. A patient who decides to have a child under these conditions must consult an oncologist and undergo an examination by a geneticist.
Research results
- The results of the studies indicate that serious adverse events such as colitis, septic shock and death remain rare over 20 years of use of the drug (approximately 600,000 patients were treated during this period), their incidence is 1 case per 10,000 patients.
- An analysis of reports to French pharmacovigilance identified 47 deaths associated with enterocolitis or septic shock between 1996 and February 2022. These data were transferred to the European Medicines Agency.
Docetaxel concentrate for the preparation of solution for inf 10 mg/ml fl 8 ml
Docetaxel concentrate for preparation of solution for inf 10 mg/ml vial 8 ml N 1
Release form
Concentrate for the preparation of solution for infusion
Compound
- 1 ml of concentrate contains
- active ingredient: docetaxel (anhydrous) 10.0 mg;
- excipients: polysorbate 80 80,000 mg, macrogol 300 648,000 mg, anhydrous citric acid 4,000 mg, ethanol 96% 275,900 mg.
Package
2 ml - colorless glass bottles (1, 5, 10) - cardboard packs. 8 ml - colorless glass bottles (1, 5, 10) - cardboard packs. 16 ml - colorless glass bottles (1, 5, 10) - cardboard packs.
pharmachologic effect
Antitumor drug of plant origin (from the taxoid group). It accumulates tubulin in microtubules and prevents their disintegration, which disrupts the mitosis phase and interphase processes in tumor cells. Docetaxel remains in cells for a long time, where its concentration reaches high values. It is active against some, although not all, cells that produce excess amounts of P-glycoprotein, which is encoded by the gene for multiple resistance to chemotherapeutic drugs.
Docetaxel Sandoz, indications for use
As a first-line treatment for breast cancer, as well as in case of ineffective therapy with anthracycline drugs; non-small cell lung cancer (including when other antitumor drugs are ineffective); malignant tumors of the head and neck; ovarian cancer.
Contraindications
Neutropenia less than 1500/μl, history of hypersensitivity to docetaxel, pregnancy, lactation.
Directions for use and doses
They are set individually, depending on the indications and stage of the disease, the state of the hematopoietic system, and the antitumor therapy regimen.
Use during pregnancy and breastfeeding
Contraindicated for use during pregnancy and lactation.
Women of childbearing potential should use reliable methods of contraception during docetaxel therapy.
Side effects
From the hematopoietic system: neutropenia, thrombocytopenia, anemia.
From the digestive system: nausea, vomiting, diarrhea, increased levels of transaminases and bilirubin in the blood serum; rarely - stomatitis.
From the nervous system: paresthesia, hyperesthesia.
From the musculoskeletal system: arthralgia, myalgia.
Allergic reactions: possible skin manifestations; rarely - bronchospasm.
Dermatological reactions: skin rash, mainly in the area of the feet, palms, as well as in the area of the upper limbs, face, chest, often accompanied by itching, sometimes followed by desquamation; hypo- or hyperpigmentation of nails and onycholysis; alopecia.
From the side of water-electrolyte metabolism: fluid retention in the body, incl. ascites, peripheral edema.
From the cardiovascular system: arterial hypotension, heart rhythm disturbances.
special instructions
Docetaxel is not used when the bilirubin content exceeds the ULN in combination with the activity of liver transaminases exceeding the ULN by 1.5 times and alkaline phosphatase exceeding the ULN by 2.5 times.
Before starting therapy, patients are prescribed GCS orally. The likelihood of developing allergic reactions and the occurrence of edema increases significantly in patients who have not previously undergone such therapy.
During the treatment period, systematic monitoring of the peripheral blood picture is necessary in order to identify the degree of myelodepression.
Drug interactions
In vitro studies have shown that the biotransformation of the drug may be altered by the simultaneous use of other drugs that induce, inhibit or are metabolized by the cytochrome CYP3A isoenzyme, such as cyclosporine, terfenadine, ketoconazole, erythromycin and troleandomycin. In this regard, caution must be exercised when using such drugs simultaneously, given the possibility of significant interactions.
When docetaxel is used simultaneously with inhibitors of the CYP3A4 isoenzyme, the risk of developing its adverse reactions may increase. If it is necessary to co-administer docetaxel with strong inhibitors of the CYP3A4 isoenzyme (ketoconazole, itraconazole, clarithromycin, indinavir, nefadozone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole), caution should be exercised and docetaxel dosage adjustment is required.
Studies conducted in patients concomitantly receiving docetaxel and ketoconazole showed that the clearance of docetaxel was reduced by 49%, apparently due to the fact that the main route of metabolism of docetaxel is its metabolism via the CYP3A4 isoenzyme. In this case, even with the use of lower doses of docetaxel, its tolerability may deteriorate.
In vitro, drugs that bind strongly to plasma proteins, such as erythromycin, diphenhydramine, propranolol, propafenone, phenytoin, salicylates, sulfamethoxazole and valproic acid, did not affect the binding of docetaxel to plasma proteins. Dexamethasone also does not affect the degree of binding of docetaxel to plasma proteins. Docetaxel does not affect the plasma protein binding of digitoxin. The pharmacokinetics of docetaxel, doxorubicin and cyclophosphamide did not change when used together.
The pharmacokinetics of docetaxel in the presence of prednisone have been studied in patients with metastatic prostate cancer, although docetaxel is metabolized by CYP3A4 and prednisone is a CYP3A4 inducer, there was no statistically significant effect of prednisone on the pharmacokinetics of docetaxel.
There is information about the interaction of docetaxel and carboplatin. When using a combination of carboplatin and docetaxel, the clearance of carboplatin increases by 50% compared with carboplatin monotherapy.
Overdose
Symptoms: bone marrow suppression, peripheral neuropathy and inflammation of the mucous membranes.
Treatment: hospitalization of the patient, careful monitoring of the functions of vital organs, prophylactic use of G-CSF, symptomatic therapy. There is currently no known antidote for docetaxel.
Storage conditions
In a place protected from light, at a temperature not exceeding 25 ° C.
Keep out of the reach of children.
Best before date
2 years.
Safety Warnings
- It should be noted that docetaxel and paclitaxel occupy an important place in the treatment of cancer and can significantly reduce mortality.
- ANSM strongly recommends that specialists and patients pay attention to the development of such side effects as neutropenia, enterocolitis, neuropathy and hypersensitivity reactions.
At the same time, the European Pharmacological Regulatory Authority (PRAC) notes that in the last 2 years there has been no increase in the frequency of side effects during docetaxel therapy; in addition, all adverse events recorded in recent years were known earlier. However, a re-discussion of the results is expected in the near future drug safety studies.
It is important to note that French pharmacovigilance plans to study the incidence of adverse events with paclitaxel, which is an alternative to docetaxel in the treatment of breast cancer.
Source:
ANSM. Docetaxel: levée de la recommandation d'éviter son utilisation dans le cancerdu sein et renforcement de l'encadrement des pratiques.
Docetaxel
Treatment with Docetaxel should only be carried out under the supervision of a physician experienced in administering antitumor chemotherapy in a specialized hospital setting.
To prevent hypersensitivity reactions, as well as to reduce fluid retention, all patients receiving Docetaxel (except for patients with prostate cancer, recommendations for premedication for which see below), in the absence of contraindications, before its administration, are premedicated with a glucocorticosteroid, for example, dexamethasone orally at a dose of 16 mg/day (8 mg twice daily) for 3 days, starting 1 day before Docetaxel administration.
In patients with prostate cancer receiving concomitant treatment with prednisone or prednisolone, premedication with dexamethasone at a dose of 8 mg is performed 12, 3 and 1 hour before the start of Docetaxel administration.
To reduce the risk of hematological complications, prophylactic administration of granulocyte colony-stimulating factor (G-CSF) is recommended.
The drug Docetaxel is administered intravenously over 1 hour once every 3 weeks.
Breast cancer (BC)
Adjuvant therapy
For adjuvant therapy of operable breast cancer with regional lymph node involvement and operable breast cancer without regional lymph node involvement, the recommended dose of Docetaxel is 75 mg/m2 1 hour after administration of doxorubicin (50 mg/m2) and cyclophosphamide (500 mg/m2) every 3 weeks ( TAS scheme). A total of 6 cycles (see also further “Dose adjustment during chemotherapy”).
For adjuvant treatment of patients with operable breast cancer with tumor overexpression of HER2, the following doses of Docetaxel are recommended.
Chemotherapy according to the AS TN regimen
- AS (cycles 1-4): doxorubicin (A) 60 mg/m2 followed by cyclophosphamide (C) 600 mg/m2 every 3 weeks, 4 cycles.
- TN (cycles 5-8): docetaxel (T) 100 mg/m2 once every 3 weeks, 4 cycles and trastuzumab (N), administered weekly according to the following regimen:
- cycle 5 (starts 3 weeks after the last AC cycle):
day 1: trastuzumab 4 mg/kg (loading dose),
day 2: docetaxel 100 mg/m2,
days 8 and 15: trastuzumab 2 mg/kg;
— cycles 6-8:
day 1: docetaxel 100 mg/m2 and trastuzumab 2 mg/kg,
days 8 and 15: trastuzumab 2 mg/kg.
- Three weeks after day 1 of cycle 8: trastuzumab 6 mg/kg every 3 weeks. Trastuzumab is administered for a total of 1 year.
Locally advanced or metastatic breast cancer
For locally advanced or metastatic breast cancer, docetaxel 75 mg/m2 is administered in combination with doxorubicin 50 mg/m2 as first-line therapy; As a 2nd line therapy, the recommended dose of docetaxel in monotherapy is 100 mg/m2.
For the combination of Docetaxel plus trastuzumab, the recommended dose of Docetaxel is 100 mg/m2 every 3 weeks with weekly administration of trastuzumab. The initial intravenous infusion of docetaxel is given the day after the first dose of trastuzumab. Subsequent doses of docetaxel are administered immediately after completion of the intravenous infusion of trastuzumab (if the previous dose of trastuzumab is well tolerated). For information on dosage and route of administration of trastuzumab, see the trastuzumab prescribing information.
When combined with capecitabine, the recommended dose of docetaxel is 75 mg/m2 every 3 weeks, and capecitabine is 1250 mg/m2 orally twice daily (within 30 minutes after meals) for 2 weeks followed by a one-week rest period. To calculate the dose of capecitabine according to body surface area, see the capecitabine prescribing information.
Non-small cell lung cancer
In patients who have not previously received chemotherapy, the following treatment regimen is recommended: docetaxel 75 mg/m2 over 30-60 minutes or carboplatin (AUC 6 mg/ml/min) over 30-60 minutes.
For treatment after failure of platinum-based chemotherapy, docetaxel monotherapy at a dose of 75 mg/m2 is recommended.
Metastatic ovarian cancer
For 2nd line treatment of ovarian cancer, a docetaxel dose of 100 mg/m2 every 3 weeks in monotherapy is recommended.
Prostate cancer
For the treatment of patients with prostate cancer, the recommended dose of Docetaxel is 75 mg/m2 once every 3 weeks. Prednisone or prednisolone is prescribed for a long time at 5 mg orally 2 times a day.
Stomach cancer
For the treatment of gastric cancer, the recommended dose of Docetaxel is 75 mg/m2 as a one-hour infusion followed by cisplatin 75 mg/m2 over 1-3 hours (both drugs only on the first day of each chemotherapy cycle). Upon completion of the administration of cisplatin, a 24-hour infusion of fluorouracil 750 mg/m2/day is carried out for 5 days. Treatment is repeated every 3 weeks. Patients should receive premedication with antiemetics and appropriate fluid supplementation (hydration) when receiving cisplatin. To reduce the risk of hematological toxicity (see below, “Dose adjustment during chemotherapy”), administration of G-CSF is indicated for prophylactic purposes.
Head and neck cancer
Patients should be premedicated with antiemetics and given adequate hydration (before and after cisplatin administration). The development of neutropenic infections should be prevented. All patients receiving treatment regimens containing Docetaxel received prophylactic antibiotics.
Induction chemotherapy followed by radiation therapy.
For induction therapy for locally advanced unresectable squamous cell carcinoma of the head and neck, the recommended dose of Docetaxel is 75 mg/m2 as a one-hour intravenous infusion followed by cisplatin at a dose of 75 mg/m2 over 1 hour (both drugs are administered only on the first day of each chemotherapy cycle). After this, a continuous intravenous infusion of fluorouracil is carried out for 5 days. This regimen is repeated every 3 weeks for 4 cycles. After chemotherapy, patients should undergo radiation therapy.
Induction chemotherapy followed by chemoradiotherapy
For induction therapy of locally advanced squamous cell carcinoma of the head and neck (technically unresectable, with a low likelihood of surgical cure, or with a decision to preserve the organ), the recommended dose of Docetaxel is 75 mg/m2 as a one-hour intravenous infusion followed by a 0.5 to 3-hour intravenous infusion an infusion of cisplatin 100 mg/m2 (both drugs are administered only on the first day of each chemotherapy cycle) followed by a continuous intravenous infusion of fluorouracil at a dose of 1000 mg/m2/day from days 1 to 4. This treatment regimen is repeated every 3 weeks for a total of 3 cycles. After chemotherapy, patients should undergo chemoradiotherapy. For information about dosage adjustments of cisplatin and fluorouracil, see the instructions for use of these drugs.
Dose adjustment during chemotherapy
General recommendations
Docetaxel should be administered when the peripheral blood neutrophil count is ≥1500/µl. In the case of febrile neutropenia, a decrease in the number of neutrophils < 500 / μl for more than one week, severe or cumulative (increased with repeated administrations) skin reactions, or severe peripheral neuropathy during docetaxel therapy, its dose for subsequent administrations should be reduced from 100 mg /m2 to 75 mg/m2 and/or from 75 mg/m2 to 60 mg/m2. If such reactions persist even at a docetaxel dose of 60 mg/m2, treatment should be discontinued.
Combination therapy including the drug Docetaxel for the treatment of breast cancer
Adjuvant therapy for breast cancer
For patients with breast cancer receiving adjuvant therapy with Docetaxel in combination with doxorubicin and cyclophosphamide (TAC regimen), G-CSF is recommended for primary prevention. For patients who have experienced febrile neutropenia or a neutropenic infection, the dose of Docetaxel should be reduced to 60 mg/m2 in all subsequent cycles. In patients who have developed grade 3 or 4 stomatitis, it is necessary to reduce the dose of docetaxel to 60 mg/m2.
The drug Docetaxel in the chemotherapy regimen AS TN
In operable breast cancer with tumor overexpression of HER2 after an episode of febrile neutropenia or infection during adjuvant therapy according to the AS TN regimen, it is necessary to use G-CSF for prophylactic purposes in all subsequent cycles, and the dose of Docetaxel in the AS TN regimen should be reduced from 100 mg /m2 up to 75 mg/m2.
Since in clinical practice the development of neutropenia was observed during the first cycle of chemotherapy, the neutropenic risk and currently generally accepted recommendations should be taken into account and, if necessary, G-CSF should be used.
In the case of stomatitis of grade 3 or 4 severity, the dose of Docetaxel in the AS TN regimen should be reduced from 100 mg/m2 to 75 mg/m2.
To adjust the dose of trastuzumab, see the information in the trastuzumab prescribing information.
Docetaxel in combination with capecitabine
To adjust the dose of capecitabine when combined with Docetaxel, see the instructions for medical use of capecitabine.
When using Docetaxel in combination with capecitabine, if grade 2 toxicity occurs for the first time and persists at the start of the next cycle, the next treatment cycle may be delayed until the toxicity is reduced to grade 0 to grade 1, with 100% of the original dose administered during the next treatment cycle. doses. In patients with recurrent grade 2 toxicity or first grade 3 toxicity at any time during the cycle, treatment is delayed until toxicity has decreased to grade 0-1, then treatment with Docetaxel is resumed at a dose of 55 mg/m2.
If any subsequent toxicity occurs or any grade 4 toxicity occurs, docetaxel should be discontinued.
Combination therapy, including the drug Docetaxel, for non-small cell lung cancer, the drug Docetaxel in combination with cisplatin or carboplatin
In patients who initially received docetaxel 75 mg/m in combination with cisplatin or carboplatin and whose platelet count in the previous cycle decreased to 25,000/μL, or in patients who developed febrile neutropenia, or in patients with severe non-hematological toxicity , the dose of docetaxel in subsequent cycles should be reduced to 65 mg/m2.
To adjust the dose of cisplatin, see the instructions for use of cisplatin.
Docetaxel in combination with cisplatin and fluorouracil for gastric cancer and head and neck cancer
Patients receiving Docetaxel in combination with cisplatin and fluorouracil should receive antiemetics and adequate fluid supplementation (hydration) in accordance with current generally accepted recommendations. To reduce the risk of complicated neutropenia, G-CSF should be used.
If, despite taking G-CSF, episodes of febrile neutropenia, prolonged neutropenia or neutropenic infection occur, the dose of Docetaxel is reduced from 75 to 60 mg/m2. With the subsequent development of episodes of complicated neutropenia, it is recommended to reduce the dose of Docetaxel from 60 mg/m2 to 45 mg/m2. With the development of grade 4 thrombocytopenia, it is recommended to reduce the dose of Docetaxel from 75 mg/m2 to 60 mg/m2. Subsequent cycles with docetaxel are possible when neutrophil counts > 1500/µl and platelet counts > 100,000/µl. If these toxic manifestations persist, treatment should be discontinued.
Recommended dose adjustments for toxicity in patients receiving Docetaxel in combination with cisplatin and fluorouracil (FU):
| Toxicity | Correction of dosage regimen |
| Diarrhea grade 3 | First episode: reduce FU dose by 20%. Repeated episode: reduce Docetaxel dose by 20%. |
| Diarrhea grade 4 | First episode: reduce the dose of Docetaxel and FU by 20%. Repeated episode: stop treatment. |
| Stomatitis/mucositis grade 3 | First episode: reduce FU dose by 20%. Repeated episode: Stop FU only in all subsequent cycles. Third episode: reduce the dose of Docetaxel by 20%. |
For recommendations on dosage adjustments of cisplatin and fluorouracil, see their prescribing information.
In patients with head and neck squamous cell carcinoma who develop complicated neutropenia (including prolonged neutropenia, febrile neutropenia, or infection), prophylactic use of G-CSF is recommended in all subsequent cycles (eg, days 1 to 15 of the cycle).
Special patient groups
Children
The safety and effectiveness of Docetaxel in children have not been studied. There is limited experience with docetaxel in children. The effectiveness and safety of Docetaxel for nasopharyngeal cancer in children and adolescents from 1 month to 18 years have not yet been established. Docetaxel has not been used in children for the following indications: breast cancer, non-small cell lung cancer, prostate cancer, gastric cancer and head and neck cancer, with the exception of poorly differentiated nasopharyngeal cancer (types I and II).
Elderly patients
Based on population pharmacokinetic analysis data, there are no special instructions for the use of Docetaxel in the elderly. In patients 60 years of age and older, when Docetaxel is combined with capecitabine, a 25% reduction in the dose of capecitabine is recommended (see instructions for use of capecitabine).
Patients with liver failure
Based on the pharmacokinetic data obtained for docetaxel in monotherapy at a dose of 100 mg/m2, in patients with ALT and/or AST activity > 1.5 ULN or alkaline phosphatase activity > 2.5 ULN, the recommended dose of Docetaxel is 75 mg/m2 . In patients with an increase in the concentration of bilirubin in the blood (> 1 ULN) and/or with an increase in the activity of ALT and AST (> 3.5 ULN) in combination with an increase in the activity of alkaline phosphatase (> 6 ULN), a dose reduction cannot be recommended and not Docetaxel should be used without strict indications. The combination of Docetaxel with cisplatin and fluorouracil in the treatment of patients with gastric cancer was not used in patients with increased ALT and/or AST activity (> 1.5 ULN) in combination with increased alkaline phosphatase activity (> 2.5 ULN) and increased bilirubin concentrations in the blood (> 1 ULN).
In such patients, no dose reduction can be recommended and docetaxel should not be used unless strictly indicated.
There are currently no data regarding the use of Docetaxel in combination with other drugs in patients with impaired liver function.
Patients with impaired renal function
There are no data on the use of docetaxel in patients with severe renal impairment.
Preparation of infusion solution
Concentrate for preparing a solution for infusion 20 mg/0.5 ml: the actual content in the bottle is 24.4 mg/0.61 ml, which allows you to compensate for the loss of liquid when preparing a pre-mixed solution caused by foaming, adhesion to the walls of the bottle and the presence of “dead” space." Thus, excess drug in the vial ensures that after reconstitution of its contents with the supplied diluent, the minimum volume of premixed solution drawn up will be 2 ml containing 10 mg/ml docetaxel, corresponding to 20 mg (the dose indicated on the vial label).
Concentrate for solution for infusion 80 mg/2 ml: actual content in the vial is 94.4 mg/2.36 ml, which allows you to compensate for liquid losses when preparing a pre-mixed solution due to foaming, adhesion to the walls of the vial and the presence of “dead space” . Thus, the excess drug in the vial ensures that, after diluting its contents with the supplied diluent, the minimum volume of the premixed solution drawn up will be 8 ml containing 10 mg/ml docetaxel, corresponding to 80 mg (the amount indicated on the vial label),
a) Preparation of a pre-mixed solution of Docetaxel (with a docetaxel concentration of 10 mg/ml)
The concentrate for preparing a solution for infusion of the drug Docetaxel must first be diluted in the supplied solvent. If the bottles with the drug and solvent were stored in the refrigerator, then before dilution they must be kept at room temperature (below 25 ° C) for 5 minutes. The entire contents of the bottle with the solvent are drawn under aseptic conditions using a needle into a syringe (the bottle is placed slightly at an angle) and injected into the bottle with Docetaxel.
After removing the needle, the contents of the bottle with the resulting mixture are mixed by turning the bottle upside down for 45 s (do not shake!) and left for 5 minutes at room temperature, after which the solution is checked for homogeneity and transparency (the presence of foam even after 5 minutes is the norm from - for the content of polysorbate 80 in the composition of the drug). The premix contains docetaxel at a concentration of 10 mg/ml and should be used immediately to prepare an infusion solution.
b) Preparation of solution for infusion. The required volume of pre-mixed solution according to the required dose is injected into an infusion bag or bottle containing 250 ml of 5% dextrose solution or 0.9% sodium chloride solution. If the required dose of docetaxel exceeds 200 mg, it should be diluted in a larger volume of solution for infusion so that the docetaxel concentration does not exceed 0.74 mg/ml. The contents of the infusion bag or bottle should be mixed using a rotational motion. Infusion of the resulting solution should be carried out no later than 4 hours after preparation (including 1 hour of administration) when stored at room temperature (below 25 ° C) and normal lighting conditions. Docetaxel premixed solution and solution for infusion, like any other parenteral drug, must be inspected before administration; If there is sediment, the solution should be destroyed.
Residues of the drug and all materials used for its dilution and administration should be disposed of in accordance with standard regulations.