Omnic
Omnic (INN tamsulosin) is an alpha-1-blocker used for the correction of dysuric disorders in prostate adenoma from the Dutch pharmaceutical company Astellas Pharma Europe. Available in capsules. Selectively inactivates alpha-1 receptors located in the smooth muscle tissue of the pelvic organs - prostate, cervical bladder, prostatic area of the urethra. By reducing the excess tone of the smooth muscle “framework” of these tissue structures, normal evacuation of urine from the body through the urinary tract becomes possible. At the same time, obstructive manifestations and symptoms of irritation characteristic of prostate adenoma are leveled out. The therapeutic effect of the drug develops, as a rule, at the end of the second week from the start of the pharmacotherapeutic course. Omnic is a selective drug that interacts only to a small extent with the B subtype of alpha-1 adrenergic receptors, so it has virtually no effect on the level of systemic blood pressure, both in hypertensive patients and in individuals with normal initial blood pressure levels. After oral administration, Omnic is quickly and completely absorbed from the digestive tract. Peak plasma concentrations after taking the recommended single dose are achieved at 7 hours of tamsulosin's presence in the body. Metabolism of the active substance is slow. The half-life of the drug is 10 hours, and with regular course use it is 13 hours.
Excreted along with urine. Taken once a day after the morning meal. Side effects are relatively rare. These include vertigo, subjective tachycardia, headache, decreased appetite, and retrograde ejaculation. Contraindication to use is individual intolerance to tamsulosin. The drug should be used with caution in persons with liver disease and high blood pressure. The use of Omnic involves preliminary diagnosis to exclude diseases that have symptoms similar to prostate adenoma. Diagnostic testing includes rectal palpation and a blood test for prostate-specific antigen. In patients with kidney disease, no dose adjustment of Omnic is required. During treatment, activities that require attention and concentration should be minimized. Furosemide, when used together, somewhat suppresses the activity of Omnic, while cimetidine, on the contrary, increases it. Diclofenac accelerates the elimination of Omnic from the body. When using the drug together with other drugs from the alpha-1-blocker group, the antihypertensive effect may be enhanced, up to the point of fainting. At the first such signs, the patient should take a sitting or lying position and wait out the developed precollapse symptoms. Data on overdose with Omnic have not been reported in the medical literature to date.
- Magazine archive /
- 2017 /
The effectiveness of the use of drugs Omnic and Omnic Ocas in patients with lower urinary tract symptoms due to benign prostatic hyperplasia (multicenter observational program)
DOI: https://dx.doi.org/10.18565/urology.2017.5.42-47
BOO. Shalekenov, E.A. Kuandykov, S.B. Shalekenov
JSC "Kazakh Medical University of Continuing Education", Department of Urology and Andrology, Almaty, Kazakhstan
The purpose of this study was to examine the effectiveness and safety of 6-month use of Omnic and Omnic Ocas in patients with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) in routine urological practice in Kazakhstan. Design. The design of the project was a multicenter prospective observational program that involved the collection of data from patients with symptoms of urinary dysfunction due to BPH who received Omnic Ocas or Omnic as primary therapy. Materials and methods. The program included 1513 patients with a verified diagnosis of LUTS/BPH who were treated with Omnic Ocas or Omnik (tamsulosin) by urological specialists in medical institutions in the Republic of Kazakhstan. Study According to the protocol, 1381 patients completed the study. The average age of the patients was 63 years. The study program included three control visits: visit 1 (initial), during which the patient filled out the I-PSS questionnaire on symptoms and quality of life, determined the maximum urine flow rate (Qmax) according to uroflowmetry and prostate volume according to digital rectal study, the level of PSA in the blood plasma was assessed. Visit 2 and Visit 3 were carried out on average 3 and 6 months, respectively, after Visit 1 and reflected the assessment of the outcomes of the prescribed therapy during the observation period of the patient. Results. During the 6-month treatment, an improvement in clinical symptoms was noted on the I-PSS scale for patients of different age groups. The drug therapy used was more effective for patients with urine flow Discussion. The results of a significant improvement in urinary function in all patients with lower urinary tract symptoms and benign prostatic hyperplasia who took part in the study according to the protocol and received therapy with Omnic Ocas or Omnic (tamsulosin) (n=1381) indicate the following: in age groups “under 55 years”, “55–65 years” and “over 65 years” a decrease in symptoms was observed (the total score on the I-PSS scale after 6 months of therapy decreased by 41.6%), the feeling of incomplete emptying of the bladder after urination was reduced, the need to urinate more often than 2 hours after urination is reduced, and a decrease in the intermittency of the stream is noted. The therapy was well tolerated: adverse events were reported in 1.6% of patients. Thus, we can conclude that the drugs Omnik and Omnik Okas are highly effective in all age groups of patients with LUTS/BPH and the favorable safety profile of these drugs in routine clinical practice. Conclusion. The data obtained allow us to conclude that the drugs Omnik and Omnik Okas are highly effective in all age groups of patients with LUTS/BPH and the favorable safety profile of these drugs.
Key words: symptoms of urinary dysfunction, benign prostatic hyperplasia, tamsulosin, controlled absorption system for oral administration, α1-adrenergic receptors
Read the article in the Doctor's Library
Literature
1. McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793–803.
2. Singh I., Agarwal V., Garg G. Tamsulosin and Darifenacin Versus Tamsulosin Monotherapy for BPH with Accompanying Overactive Bladder. J. Clin Diagnosis Res. 2015;9(6):PC08–11.
3. Yuan JQ, Mao C., Wong SY, Yang ZY, Fu XH, Dai XY, Tang JL Comparative Effectiveness and Safety of Monodrug Therapies for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Network Meta-analysis. Medicine (Baltimore). 2015;94(27):e974.
4. Bishr M., Boehm K., Trudeau V., Tian Z., Dell'Oglio P., Schiffmann J., Jeldres C., Sun M., Shariat SF, Graefen M., Saad F., Karakiewicz PI Medical management of benign prostatic hyperplasia: Results from a population-based study. Can Urol Assoc J 2016;10(1-2):55–59.
5. Carnevale FC, Antunes AA, da Motta Leal Filho JM et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovasc Intervent Radiol. 2010;33(2):355–361.
6. Michel MC et al. Cardiovascular Safety of the Oral Controlled Absorption System (OCAS) Formulation of Tamsulosin Compared to the Modified Release (MR) Formulation. Eur Urol. 2005;4(2 Suppl):53–60.
7. Chapple CR et al. Tamsulosin Oral Controlled Absorption System (OCAS) in Patients with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia (LUTS/BPH): Efficacy and Tolerability in a Placebo and Active Comparator Controlled Phase 3a Study. Eur Urol. 2005;4(2 Suppl):25–32.
8. Yamada S., Suzuki M., Tanaka C. et al. Comparative study on alpha 1-adrenoceptor antagonist binding in human prostate and aorta. Clin Exp Pharmacol Physiol. 1994;21:405–411.
9. Shatylko TV Using uroflowmetry for diagnosis and evaluation of the effectiveness of treatment of urological diseases. Byulleten' meditsinskikh internet-konferentsii. 2012;2(2):137. Russian (Shatylko T.V. The use of uroflowmetry in the diagnosis and assessment of the effectiveness of treatment of urological diseases. Bulletin of medical Internet conferences. 2012;2(2):137).
About the authors / For correspondence
AUTHORIZATION: B. U Shalekenov – Doctor of Medical Sciences, Professor, Head. Department of Urology and Andrology JSC “Kazakh Medical University of Continuing Education” Almaty, Kazakhstan; e-mail
OMNIK (capsules)
Back in another part of the country, the clinic told us that there was a similar drug with a similar effect and name.
And it was better for us to buy the second one... But by that time we had almost completed the course; nothing could be changed. I am writing this review from the point of view of the owners who observe the effect of this drug. Alas, the animal cannot say what it feels and how, so I want to write about what we observed. I hope the information is useful to you.
It is not difficult to buy the drug, you can do it without a prescription, but it was not available in every pharmacy. The cost is approximately 140 UAH (about 330 rubles). The box looks like this:
There is no special information on the sides. Be sure to check the expiration date.
The instructions are very large. But I advise everyone to read it, since the side effects are strong, and you need to know what will happen to you (your family). You can read part of the instructions in the photo:
There are no particular difficulties in application. We tried to give 1 capsule after breakfast at approximately the same time. I had to be careful about swallowing the medicine, since the capsule cannot be split.
Externally, the capsules differ from others, and therefore it is difficult to confuse them. The blister with them looks like this:
And the flip side:
Our application. We began to give this medicine as prescribed by the doctor during the disease crisis. On the 2nd day, it was easier for the dog to go to the toilet, but his general condition began to deteriorate. The temperature rose and did not fall. The husband decided to stop the drug after 4 days and look at the body’s reaction. The problem returned - it was impossible to urinate again. They chose the lesser of two evils and resumed the course again. The doctors who prescribed the drug said that the general poor condition of the body, as well as many possible side effects, is inevitable. But they won't be able to help with other drugs or treatments until this underlying crisis passes.
I had to wait for the entire course, about a month. The general condition was really bad. Yes, the dog began to go to the toilet easier and endure less. This became the main advantage of the medicine. But the stream was still thin and jerky, so I had to stand for a long time. We had a fever most days; the only difference was how high it was. The pet was very lethargic, he didn’t get up, let alone go for a walk. It was difficult to move his paws, so we tried, soon after breakfast, we started to have loose stools, so we calculated the time to go for a walk with him before lunch. Sometimes he refused to eat. In order not to put extra stress on the stomach and liver, we changed the diet.
Another property of the drug became no less serious. Despite the fact that it is considered an antibiotic and should kill all sorts of nasty things in the body, we had a slightly different effect. Yes, sometimes various bees, wasps, and insects tried to bite the dog; on a walk, sometimes he caught ostyuki (from a plant like a sharp grain). But often we found inflammation if it appeared and it went away quickly. And then what happened is unknown, but a lump began to form on our neck. It was getting bigger. Since at this time we often visited veterinarians, we were under supervision. The cause of the inflammation could not be determined. We were told that there was no point in opening it while we were using this drug. And at the end, “the inflammation will go away naturally.” So it actually happened that after finishing the course, on the 2nd day, an abscess opened in that place and additional treatment of the resulting wound was required. It’s hard for me to call it a coincidence, but I can’t explain the relationship either.
View the side effects indicated by the manufacturer in the table:
Bottom line. The effect after taking it lasted for a week. Then the problem returned, maybe just not with such force. I had to go to different veterinary hospitals in another city. I won’t say that the reception was in vain, but we are very glad that we no longer give it to our pet. You can read about a different drug prescribed in another review (“Prostatilen”).
Are drugs for the treatment of benign prostatic hyperplasia safe?
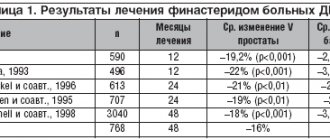
In December 2015, the Journal of Urology published the results of a new study that examined the safety of new drugs for the treatment of benign prostatic hyperplasia (BPH): doxazosin, finasteride, and their combination. The six most common side effects with their use were: dizziness, orthostatic hypotension, weakness, impotence, decreased libido and impaired ejaculation.
The study randomized 3047 men with BPH aged 50 years or older to receive placebo, doxazosin (4-8 mg), finasteride (5 mg), or doxazosin + finasteride. The follow-up period for patients averaged 4.5 years. The incidence of side effects was compared with placebo.
results
Analysis of the first year of therapy showed that the risk of developing dizziness
was significantly higher with the use of doxazosin (2.2 times; p<0.001) and the combination of drugs (2.8 times; p<0.001). After a year of treatment, only patients who were prescribed the combination of drugs were significantly more likely to report dizziness (2.1 times; p<0.001).
The risk of developing orthostatic hypotension during the first year of treatment was significantly higher when using doxazosin (2.4 times; p<0.001), as well as the combination of doxazosin and finasteride (2.7 times; p<0.001). Whereas after a year of therapy, only in patients receiving doxazosin, the incidence of orthostatic hypotension was 1.5 times higher (p=0.023).
Weakness
occurred significantly more often in patients receiving combination therapy in the first year of treatment (3.1 times; p=0.002), but then the frequency of its development was the same in the groups.
The analysis demonstrated that the incidence of impotence
in the first year of therapy was significantly higher when using finasteride (1.9 times; p=0.006) and the combination of doxazosin and finasteride (2.4 times; p <0.001). But after a year of therapy, the incidence of impotence was the same as in the placebo group.
Decreased libido
occurred significantly more often in patients receiving finasteride (1.7 times; p=0.021) and combination therapy in the first year of treatment (2 times; p=0.001), but then the frequency of its development was comparable to that in the placebo group.
Ejaculation disorder
occurred significantly more often when finasteride was prescribed (2.5 times; p=0.002) and a combination of drugs (5.5 times; p<0.001) in the first year of therapy. When using the drugs for more than a year only against the background of doxazosin + finasteride, the frequency of ejaculation disorders was increased (1.8 times; p = 0.045).
Source:
Lorraine L. Janeczko. More Side Effects in First Year With BPH Drugs. Medscape.com