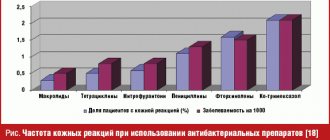
Relevance
In February 2022, the French National Medical Agency (ANSM) recommended temporarily stopping the use of docetaxel in patients with infiltrative non-metastatic breast cancer.
French pharmacovigilance has previously reported potential serious adverse events associated with the use of Taxam. Taxams (docetaxel and paclitaxel) occupy an important place in the treatment of certain malignant neoplasms, for which there is sometimes no alternative treatment method. This class of drugs, like many others, have severe side effects.
On July 5, 2022, a safety report on the drug was presented on the ANSM website.
Docetaxel Sandoz, 10 mg/ml, concentrate for solution for infusion, 16 ml, 1 pc.
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1000, < 1/100), rare (≥1/10000, <1/1000) and very rare (<1/10000); frequency unknown (the frequency of events cannot be determined from the available data).
Monotherapy (75 mg/m2 and 100 mg/m2)
Blood and lymphatic system disorders
Often:
reversible and non-cumulative (not worsening with repeated administrations) neutropenia, observed in 96.6% of patients who did not receive G-GFR. The number of neutrophils decreases to minimum values after an average of 7 days (in patients who have previously received chemotherapy, this period may be shorter), the average duration of severe neutropenia (less than 500 cells/μl) is 7 days; febrile neutropenia, anemia, thrombocytopenia, infections;
Often:
severe infections combined with a decrease in the number of neutrophils in peripheral blood less than 500/μl; severe infections, including sepsis and pneumonia, incl. with fatal outcome; thrombocytopenia less than 100,000/µl, bleeding combined with thrombocytopenia less than 50,000/µl and anemia (hemoglobin concentration less than 11 g/dl), incl. severe (hemoglobin concentration less than 8 g/dL);
Infrequently:
severe thrombocytopenia.
Immune system disorders
Often:
allergic reactions that usually occur within a few minutes of starting the infusion and are mild or moderate in severity (“flushing” of the face, rash with or without itching, chest tightness, back pain, shortness of breath, drug fever, or chills);
Often:
severe allergic reactions, characterized by a decrease in blood pressure and/or bronchospasm or generalized rash/erythema, which disappeared after stopping the intravenous infusion and carrying out appropriate therapy.
Skin and subcutaneous tissue disorders
In some cases, a combination of several factors, such as concomitant infections, concomitant therapy and the underlying disease, had an additional influence on the occurrence of these reactions.
Often:
reversible skin reactions are usually mild or moderate: localized rash, mainly on the arms and legs, as well as on the face and chest, which is often accompanied by itching, the rash usually occurred within one week after docetaxel infusion; nail disorders are characterized by hypo- and hyperpigmentation, pain and onycholysis; alopecia;
Often:
severe skin reactions, incl. rash followed by desquamation, including severe hand-foot syndrome, which may require interruption or discontinuation of docetaxel treatment;
Infrequently:
severe alopecia.
Gastrointestinal disorders
Often:
nausea, vomiting, diarrhea, anorexia, stomatitis;
Often:
severe nausea and vomiting, severe diarrhea, constipation, severe stomatitis, esophagitis, pain in the epigastric region (including severe), gastrointestinal bleeding;
Infrequently:
severe gastrointestinal bleeding, severe constipation and esophagitis.
Disorders of the liver and biliary tract
Often:
increased serum activity of AST, ALT, alkaline phosphatase and bilirubin concentration in the blood plasma (more than 2.5 times the ULN).
Nervous system disorders
Often:
mild or moderate neurosensory reactions: paresthesia, dysesthesia, pain, including a burning sensation; and neuromotor reactions, mainly manifested by muscle weakness, impaired taste;
Often:
severe neurosensory reactions and neuromotor reactions (grade 3–4);
Infrequently:
severe disturbance of taste sensations.
If these neurological symptoms occur, the dosage regimen should be adjusted.
If symptoms of neuropathy persist, treatment should be discontinued. The average time to spontaneous resolution of neurotoxic reactions was 81 days from their onset (range 1 to 741 days).
Heart disorders
Often:
heart rhythm disturbances;
Infrequently:
heart failure.
Vascular disorders
Often:
increase or decrease in blood pressure, bleeding.
Respiratory, thoracic and mediastinal disorders
Often:
dyspnea;
Often:
severe shortness of breath.
Musculoskeletal and connective tissue disorders
Often:
myalgia;
Often:
arthralgia.
General and administration site disorders
Often:
asthenia, including severe; generalized and localized pain syndrome, including chest pain of non-cardiac origin.
Fluid retention: the development of peripheral edema and weight gain and, less often, the appearance of pleural effusion and ascites have been reported. Peripheral edema usually began in the lower extremities and could become generalized with an increase in body weight of 3 kg or more. Fluid retention is cumulative (increases with repeated administrations of the drug). Fluid retention was not accompanied by acute episodes of decreased blood pressure;
Often:
reactions at the injection site, usually mild: hyperpigmentation, inflammation, redness or dryness of the skin, phlebitis, hemorrhage from a punctured vein or swelling of the vein; pronounced generalized and localized pain syndrome, including chest pain of non-cardiac origin, severe forms of fluid retention.
In patients treated with docetaxel monotherapy at a dose of 100 mg/m2, the median total dose until the end of treatment due to fluid retention was more than 1000 mg/m2, and the median time until resolution of fluid retention was 16.4 weeks (range 0 to 42). weeks). In patients receiving premedication, there was a delay in the onset of moderate to severe fluid retention (mean total doses of docetaxel at which fluid retention was observed were 818.9 mg/m2 with premedication and 489.7 mg/m2 without premedication) , however, in some cases, fluid retention developed already during the first courses of therapy.
Docetaxel Sandoz® in combination with other drugs
Docetaxel Sandoz® in combination with doxorubicin
When Docetaxel Sandoz® was used in combination with doxorubicin, a greater incidence of neutropenia, including severe neutropenia, was observed when compared with Docetaxel Sandoz® monotherapy; febrile neutropenia; thrombocytopenia, including severe thrombocytopenia; anemia; infections, including severe infections; nausea; vomiting; diarrhea, including severe diarrhea; constipation; stomatitis, including severe stomatitis; heart failure; alopecia; but lower frequency of allergic reactions; skin reactions, including severe ones; nail lesions, including severe ones; fluid retention, including severe; anorexia, neurosensory and neuromotor reactions, including severe forms; hypotension; rhythm disturbances; increased activity of liver transaminases, alkaline phosphatase, bilirubin content in the blood; myalgia; asthenia.
Docetaxel Sandoz® in combination with doxorubicin and cyclophosphamide (TAS regimen)
When using this chemotherapy regimen, compared with Docetaxel Sandoz® monotherapy, a lower incidence of neutropenia, severe anemia, febrile neutropenia, infections, allergic reactions, peripheral edema, neurosensory and neuromotor reactions, nail lesions, diarrhea, arrhythmias was observed, but a higher incidence of mild anemia, thrombocytopenia, nausea, vomiting, stomatitis, taste disturbances, constipation, asthenia, arthralgia, alopecia, colitis, enterocolitis, myelodysplastic syndrome.
Additionally, the following were observed: perforation of the large intestine without lethal outcomes (2 out of 4 patients required discontinuation of treatment), acute myeloid leukemia/myelodysplastic syndrome.
Prophylactic use of G-CSF reduced the incidence of neutropenia (by 60%) and grade 3–4 neutropenic infections.
During a long-term observation period (10 years and 5 months), the following were noted: 3 cases of congestive heart failure; 1 case of dilated cardiomyopathy with fatal outcome; alopecia (including with increased manifestation of alopecia during observation), amenorrhea, asthenia, lymphatic edema, peripheral edema, peripheral sensory neuropathy. Most persistent adverse events resolved during the observation period.
Doxorubicin and cyclophosphamide followed by Docetaxel Sandoz® in combination with trastuzumab (AS TN regimen)
Alopecia was more common with these chemotherapy regimens compared with Docetaxel Sandoz® monotherapy; anemia, including grade 3–4 anemia; thrombocytopenia, including grade 3–4 thrombocytopenia; nausea, including grade 3–4 nausea; stomatitis; vomit; diarrhea; constipation; anorexia; stomach ache; increased activity of AST, ALT and alkaline phosphatase; myalgia; nail damage; arthralgia; infections of 3–4 severity; heart failure. There was no increase in febrile neutropenia. Less common were neutropenia grades 3–4, fluid retention, neurosensory and neuromotor reactions, rash and desquamation, and allergic reactions. Additionally, insomnia and increased creatinine concentration in the blood were registered.
Docetaxel Sandoz® in combination with capecitabine
When using Docetaxel Sandoz® in combination with capecitabine, a more frequent development of adverse events from the gastrointestinal tract (stomatitis, diarrhea, vomiting, constipation, abdominal pain, taste disturbances) is observed; arthralgia; severe thrombocytopenia and anemia; hyperbilirubinemia; palmar-plantar syndrome (hyperemia of the skin of the extremities (palms and feet) followed by swelling and desquamation); but more rare development of severe neutropenia; alopecia; nail disorders, changes in nail color, including onycholysis, shortness of breath, paresthesia, dehydration, lacrimation; asthenia; myalgia; decreased appetite and anorexia.
Additionally, dyspepsia, dry mouth, sore throat, oral candidiasis, dermatitis, erythematous rash, pyrexia, pain in the limbs, back pain, lethargy (drowsiness, lethargy, stupor), cough, nosebleeds, dizziness, headache, peripheral neuropathy, weight loss.
Compared with younger patients, patients 60 years of age and older receiving the combination of Docetaxel Sandoz® and capecitabine were more likely to develop grade 3-4 toxicities.
Docetaxel Sandoz® in combination with trastuzumab
In patients receiving a combination of Docetaxel Sandoz® with trastuzumab (compared to monotherapy with Docetaxel Sandoz®, nausea, diarrhea, constipation, abdominal pain, taste disturbances, febrile neutropenia, arthralgia, anorexia, grade 4 toxic effects, cases of heart failure, especially in patients previously treated with anthracyclines as adjuvant therapy, but grade 3–4 neutropenia, asthenia, weakness, alopecia, nail damage, skin rashes, vomiting, stomatitis and myalgia were less common.
Additionally observed: lacrimation, conjunctivitis, pain, shortness of breath, paresthesia, inflammation of the mucous membranes, nasopharyngitis, pain in the pharynx and larynx, nosebleeds, rhinorrhea, influenza-like illnesses, cough, pyrexia, chills, chest pain, pain in the extremities, pain in the back, bone pain, lethargy (drowsiness, lethargy, numbness), insomnia, erythema, dyspepsia, headache, hypoesthesia. Compared with docetaxel monotherapy, an increase in the incidence of severe adverse reactions was observed.
Combination of Docetaxel Sandoz® with cisplatin or carboplatin
When using these chemotherapy regimens, compared with monotherapy with Docetaxel Sandoz®, thrombocytopenia occurred more often, including grade 3–4 thrombocytopenia (more so with carboplatin); anemia, including grade 3–4 anemia; nausea, including grade 3–4 nausea; diarrhea of 3–4 severity; anorexia (more so when using cisplatin); reactions at the injection site. However, neutropenia, including grade 3–4 neutropenia, was observed less frequently; infections; febrile neutropenia; allergic reactions; skin reactions; nail damage; fluid retention, including grade 3–4 fluid retention (more so with carboplatin); stomatitis, neurosensory and, to a lesser extent, neuromotor neuropathy; alopecia; asthenia and myalgia.
Additionally, the following were observed: fever in the absence of infection, including grade 3–4; pain.
Combination of Docetaxel Sandoz® with prednisolone or prednisone
When using Docetaxel Sandoz® in combination with prednisone or prednisone, compared with monotherapy with Docetaxel Sandoz®, the incidence of side effects was significantly reduced: anemia, including grade 3–4 severity; infections; neutropenia, including grades 3–4; thrombocytopenia; febrile neutropenia; weaknesses; allergic reactions; neurosensory and neuromotor reactions; alopecia; rash; desquamation; nausea; diarrhea; stomatitis; vomiting; anorexia; myalgia; arthralgia; fluid retention; but taste disturbance and heart failure were more common.
Additionally observed: nosebleeds, cough, weakness, lacrimation.
Combination of Docetaxel Sandoz® with cisplatin and fluorouracil
When using this combination, compared with monotherapy with Docetaxel Sandoz®, anemia was more often observed, including grades 3–4; thrombocytopenia, including grades 3–4; febrile neutropenia; neutropenic infections (even with the use of G‑CSF); nausea; vomit; anorexia; stomatitis; diarrhea; esophagitis/dysphagia/pain when swallowing; but infections were observed less frequently; allergic reactions; fluid retention; neurosensory and neuromotor reactions; myalgia; alopecia; rash; itching; nail damage; skin desquamation; rhythm disturbances.
Additionally, fever in the absence of infection was observed; lethargy (drowsiness, lethargy, numbness); hearing changes; dizziness; lacrimation; dry skin; heartburn; myocardial ischemia; emphasized venous pattern; cancer pain; weight loss.
Prophylactic administration of G-CSF reduces the incidence of febrile neutropenia and/or neutropenic infectious complications.
Post-marketing use
Benign, malignant and unspecified neoplasms (including cysts and polyps)
Very rarely:
acute myeloid leukemia and myelodysplastic syndrome associated with docetaxel when used in combination with other chemotherapeutic agents and/or radiotherapy.
Blood and lymphatic system disorders
Cases of bone marrow suppression and other hematological adverse reactions have been reported.
Cases of disseminated intravascular coagulation (DIC) have been reported, often in combination with sepsis or multiple organ failure.
Immune system disorders
Rarely:
anaphylactic shock, sometimes fatal. In patients receiving premedication, these cases were very rarely fatal;
Frequency unknown:
Potentially fatal hypersensitivity reactions to docetaxel have been reported in patients who have previously had hypersensitivity reactions to paclitaxel.
Nervous system disorders
Rarely:
convulsions and transient loss of consciousness, sometimes developing during intravenous infusion of the drug.
Visual disorders
Rarely:
lacrimation with or without conjunctivitis;
Very rarely:
cases of lacrimal duct obstruction leading to excessive lacrimation, mainly in patients simultaneously receiving other anticancer drugs. Transient visual disturbances (“flashes of light” in the eyes, the appearance of scotomas), usually occurring during intravenous infusion of the drug and combined with the development of hypersensitivity reactions, which usually disappeared after stopping the intravenous infusion.
Cases of cystoid macular edema have been reported in patients treated with docetaxel as well as other taxanes.
Hearing and labyrinth disorders
Rarely:
cases of ototoxicity of the drug with hearing impairment and/or hearing loss, including cases associated with taking other ototoxic drugs.
Vascular disorders
Rarely:
venous thromboembolic complications and myocardial infarction;
Frequency unknown:
Ventricular arrhythmias, including ventricular tachycardia, sometimes fatal, have been reported in patients receiving docetaxel in combination with doxorubicin, fluorouracil and/or cyclophosphamide.
Respiratory, thoracic and mediastinal disorders
Rarely:
acute respiratory distress syndrome, interstitial pneumonia/pneumonitis, interstitial lung disease, pulmonary fibrosis, respiratory failure, which could be fatal. Rare cases of radiation pneumonitis occur with simultaneous radiation therapy.
Gastrointestinal disorders
Rarely:
dehydration as a consequence of the development of reactions from the gastrointestinal tract, including enterocolitis and perforation of the stomach or intestines; rare cases of ileus (intestinal obstruction) and intestinal obstruction;
Frequency unknown:
Enterocolitis, including colitis, ischemic colitis and neutropenic enterocolitis, with possible death, has been reported.
Disorders of the liver and biliary tract
Very rarely:
hepatitis, sometimes fatal, mainly in patients with concomitant liver diseases.
Skin and subcutaneous tissue disorders
Cutaneous lupus erythematosus, bullous rash, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (in some cases, multiple factors contributed to the development of these conditions, such as concomitant infections, concomitant medications and concomitant diseases), scleroderma-like changes , which are preceded by lymphangiectatic edema. In some cases, multiple factors contributed to the development of these conditions, such as concomitant infections, concomitant use of other medications, and concomitant diseases;
Frequency unknown:
Cases of persistent alopecia have been reported.
General and administration site disorders
Rarely:
the phenomenon of return of local radiation reaction in a previously irradiated area, dehydration, pulmonary edema;
Frequency unknown:
after docetaxel was administered to another site, a reaction occurred at the site of the previous injection (skin reaction at the site of previous extravasation).
Renal and urinary tract disorders
Deterioration of renal function and the development of renal failure have been reported, in most cases associated with the simultaneous use of nephrotoxic drugs.
Metabolic and nutritional disorders
Cases of electrolyte imbalance have been reported: hyponatremia, mainly in combination with dehydration, vomiting and pneumonia; hypokalemia, hypomagnesemia and hypocalcemia, usually associated with gastrointestinal disorders, in particular diarrhea.
Research results
- The results of the studies indicate that serious adverse events such as colitis, septic shock and death remain rare over 20 years of use of the drug (approximately 600,000 patients were treated during this period), their incidence is 1 case per 10,000 patients.
- An analysis of reports to French pharmacovigilance identified 47 deaths associated with enterocolitis or septic shock between 1996 and February 2022. These data were transferred to the European Medicines Agency.
Safety Warnings
- It should be noted that docetaxel and paclitaxel occupy an important place in the treatment of cancer and can significantly reduce mortality.
- ANSM strongly recommends that specialists and patients pay attention to the development of such side effects as neutropenia, enterocolitis, neuropathy and hypersensitivity reactions.
At the same time, the European Pharmacological Regulatory Authority (PRAC) notes that in the last 2 years there has been no increase in the frequency of side effects during docetaxel therapy; in addition, all adverse events recorded in recent years were known earlier. However, a re-discussion of the results is expected in the near future drug safety studies.
It is important to note that French pharmacovigilance plans to study the incidence of adverse events with paclitaxel, which is an alternative to docetaxel in the treatment of breast cancer.
Source:
ANSM. Docetaxel: levée de la recommandation d'éviter son utilisation dans le cancerdu sein et renforcement de l'encadrement des pratiques.
Description of the drug DOTSETAKSEL
Treatment with docetaxel should only be carried out under the supervision of a physician experienced in administering antitumor chemotherapy in a specialized hospital setting.
Docetaxel is not used when the bilirubin content exceeds the ULN in combination with the activity of liver transaminases exceeding the ULN by 1.5 times and alkaline phosphatase exceeding the ULN by 2.5 times.
Before starting docetaxel therapy, patients are prescribed oral corticosteroids. The likelihood of developing allergic reactions and the occurrence of edema increases significantly in patients who have not previously undergone such therapy.
During the treatment period, systematic monitoring of the peripheral blood picture is necessary in order to identify the degree of myelodepression.
Clinical blood counts should be monitored in patients receiving docetaxel therapy. The maximum decrease in neutrophils occurs by day 7, but in patients who have previously undergone an intensive course of chemotherapy, this interval may be shorter. If severe neutropenia develops (<500/μl for 7 days) during a course of docetaxel, it is recommended to reduce its dose in subsequent cycles or use adequate symptomatic measures. It is possible to continue treatment with docetaxel after the neutrophil count has returned to >1500/µl.
Docetaxel should be used with caution in patients with neutropenia, especially those at risk of developing gastrointestinal complications. The development of enterocolitis is possible throughout treatment. Enterocolitis can lead to death even on the first day of its development.
In order to detect the development of a hypersensitivity reaction, patients should be carefully monitored, especially during the first and second infusion. The development of hypersensitivity reactions is possible in the very first minutes of docetaxel infusion, therefore, when administering it, it is necessary to have medications and equipment for the treatment of arterial hypotension and bronchospasm.
In patients receiving docetaxel monotherapy at a dose of 100 mg/m2 and having increased liver transaminase activity (ALT and/or AST), more than 1.5 times the ULN, in combination with an increase in alkaline phosphatase activity, more than 2.5 times above ULN, there is an extremely high risk of developing severe side effects such as sepsis, gastrointestinal bleeding, febrile neutropenia, infections, thrombocytopenia, severe toxic skin lesions including death, as well as stomatitis and asthenia. Liver function tests should be performed prior to initiation of treatment and before each subsequent cycle of docetaxel therapy.
Careful monitoring of patients with severe fluid retention is necessary:
- with effusion into the pleural cavity, pericardium or ascites. When edema appears, restriction of salt and drinking regime and the use of diuretics are indicated.
In patients 65 years of age and older treated with docetaxel every 3 weeks for prostate cancer, the incidence of nail changes, anemia, infections, anorexia, and weight loss was >10% greater than in younger patients, and in patients In those 75 years and older, the incidence of fever, diarrhea, anorexia, and peripheral edema was >10% higher than in younger patients. There were no differences in the effectiveness of therapy when comparing older and younger patients.
Men and women of childbearing potential should use reliable methods of contraception during treatment with docetaxel. Men receiving docetaxel treatment are advised to abstain from conceiving a child during docetaxel treatment and for at least 6 months after completion of chemotherapy.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, patients should avoid driving vehicles and other activities that require high concentration and speed of psychomotor reactions.