Description of the drug IBUFEN ULTRA
With simultaneous use, ibuprofen reduces the effect of antihypertensive drugs (ACE inhibitors, beta-blockers), diuretics (furosemide, hydrochlorothiazide).
When used simultaneously with anticoagulants, their effect may be enhanced.
When used simultaneously with GCS, the risk of side effects from the gastrointestinal tract increases.
When used simultaneously, ibuprofen can displace indirect anticoagulants (acenocoumarol), hydantoin derivatives (phenytoin), and oral hypoglycemic drugs, sulfonylurea derivatives, from compounds with blood plasma proteins.
When used simultaneously with amlodipine, a slight decrease in the antihypertensive effect of amlodipine is possible; with acetylsalicylic acid - the concentration of ibuprofen in the blood plasma decreases; with baclofen - a case of increased toxic effects of baclofen has been described.
When used simultaneously with warfarin, an increase in bleeding time is possible; microhematuria and hematomas were also observed; with captopril - the antihypertensive effect of captopril may be reduced; with cholestyramine - a moderate decrease in the absorption of ibuprofen.
When used simultaneously with lithium carbonate, the concentration of lithium in the blood plasma increases.
When used simultaneously with magnesium hydroxide, the initial absorption of ibuprofen increases; with methotrexate - the toxicity of methotrexate increases.
The simultaneous use of NSAIDs and cardiac glycosides can lead to worsening heart failure, a decrease in glomerular filtration rate and an increase in the concentration of cardiac glycosides in the blood plasma.
There is evidence of the likelihood of an increase in the concentration of methotrexate in the blood plasma during the use of NSAIDs.
With simultaneous use of NSAIDs and cyclosporine, the risk of nephrotoxicity increases.
NSAIDs may reduce the effectiveness of mifepristone, so taking NSAIDs should be started no earlier than 8-12 days after stopping mifepristone.
Concomitant use of NSAIDs and tacrolimus may increase the risk of nephrotoxicity.
Concomitant use of NSAIDs and zidovudine may lead to increased hematotoxicity. There is evidence of an increased risk of hemarthrosis and hematomas in HIV-positive patients with hemophilia who received concomitant treatment with zidovudine and ibuprofen.
In patients receiving concomitant treatment with NSAIDs and quinolone antibiotics, the risk of seizures may increase.
In patients receiving concomitant NSAIDs and myelotoxic drugs, hematotoxicity increases.
With the simultaneous use of ibuprofen and cefamandole, cefoperazone, cefotetan, valproic acid, plicamycin, the incidence of hypoprothrombinemia increases.
With the simultaneous use of ibuprofen and drugs that block tubular secretion, there is a decrease in excretion and an increase in plasma concentration of ibuprofen.
With the simultaneous use of ibuprofen and inducers of microsomal oxidation (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants), there is an increase in the production of hydroxylated active metabolites and an increased risk of developing severe intoxications.
Ibufen D forte 200 mg/5 ml 40 ml suspension. for oral administration with raspberry flavor bottle.
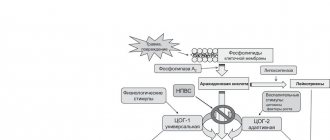
Instructions for medical use of the drug IBUFEN® D Forte Trade name Ibufen® D Forte International nonproprietary name Ibuprofen Dosage form Oral suspension, raspberry 200 mg/5 ml Composition 5 ml of suspension contain the active substance - ibuprofen 200 mg, excipients: hypromellose, xanthan gum, glycerin, sodium benzoate, liquid maltitol, sodium citrate, citric acid monohydrate, sodium saccharinate, sodium chloride, raspberry flavor: aromatic components, triacetin (E1518), water; purified water. Description A homogeneous suspension of white or almost white color with a raspberry odor. Pharmacotherapeutic group Anti-inflammatory and antirheumatic drugs. Non-steroidal anti-inflammatory drugs. Propionic acid derivatives. Ibuprofen ATC code M01AE01 Pharmacological properties Pharmacokinetics After administration, the drug is rapidly absorbed from the gastrointestinal tract (GIT) and distributed in the body. The maximum serum concentration of ibuprofen is achieved 45 minutes after administration on an empty stomach. Food reduces the absorption of ibuprofen, but does not reduce its bioavailability and tmax is 1 - 2 hours. Ibuprofen is approximately 99% bound to plasma proteins. The main proteins that bind the drug are albumin. Ibuprofen and its metabolites are quickly and completely eliminated from the body by the kidneys. The half-life of the drug is approximately 2 hours. According to limited data, ibuprofen is excreted in breast milk in very low concentrations. Pharmacodynamics Ibuprofen is a propionic acid derivative that has antipyretic, analgesic and anti-inflammatory effects. The mechanism of action of ibuprofen is based on inhibition of the synthesis and release of prostaglandins by inhibiting the activity of prostaglandin cyclooxygenase, which catalyzes the conversion of arachidonic acid to prostaglandins, but other mechanisms are not excluded. The antipyretic and analgesic effects of ibuprofen occur within 30 minutes from the moment of taking the drug. Indications for use Increased body temperature of various origins with: colds, acute respiratory viral infections, influenza, sore throat, pharyngitis, childhood infections accompanied by an increase in body temperature Pain syndrome of various origins of weak and moderate intensity with: pain in the ears with an inflammatory process in the middle ear, toothache, painful teething headaches, migraines neuralgia muscle pain pain in bones and joints due to injuries to the musculoskeletal system (damage, sprains) pain due to soft tissue injuries, postoperative pain Dosage method: Used orally. 5 ml of suspension contain 200 mg of ibuprofen. Before use, shake until a homogeneous suspension is obtained. The drug is taken after meals with plenty of liquid. For precise dosage, a syringe dispenser is included in the package. The dose is set depending on the age and body weight of the child. The usually used single dose of Ibufen® D Forte is 7-10 mg/kg body weight up to a maximum daily suspension dose of 30 mg/kg body weight. The drug is prescribed in single doses according to the scheme below: Body weight (patient's age) Single dose Maximum daily dose 10-15 kg (children from 1 to 3 years old) 2.5 ml (corresponds to 100 mg of ibuprofen) 7.5 ml (corresponds to 300 mg ibuprofen) 16-19 kg (children from 4 to 6 years old) 4 ml (corresponding to 160 mg ibuprofen) 12 ml (corresponding to 480 mg ibuprofen) 20-29 kg (children from 7 to 9 years old) 5.0 ml (corresponding to 200 mg ibuprofen) 15 ml (corresponds to 600 mg ibuprofen) 30-39 kg (children from 10 to 12 years old) 5.0-7.5 ml (corresponds to 200-300 mg ibuprofen) 22.5 ml (corresponds to 900 mg ibuprofen) More 40 kg (children and adolescents over 12 years of age and adults) 5.0-10 ml (corresponding to 200-400 mg ibuprofen) 30 ml (corresponding to 1200 mg ibuprofen) The maximum daily dose should not be exceeded. A 6-hour interval should be maintained between subsequent doses. If symptoms persist, worsen, or if new symptoms appear, the patient should consult a doctor. The drug should not be used for more than 3 days without medical supervision. The drug does not contain sugar. Side effects Common ( 1/1000 to 1/100): heartburn, abdominal pain, nausea, vomiting, diarrhea, flatulence, constipation, mild bleeding from the gastrointestinal tract, which in exceptional cases can lead to anemia Not common ( 1/ 1000 to 1/100): allergic reactions with skin rash and itching attacks of suffocation (can occur along with a decrease in blood pressure) headache, dizziness, insomnia, agitation, irritability, feeling tired visual disturbances ulceration of the gastrointestinal tract, possibly with perforation or bleeding ulcerative stomatitis , gastritis, exacerbation of colitis and Crohn's disease Rarely ( 1/10000 to 1/1000): tinnitus Very rarely ( 1/10000): hematopoietic disorders (anemia, leukopenia, thrombocytopenia, pancytopenia, agranulocytosis). necrotizing fasciitis severe hypersensitivity reactions: swelling of the face, tongue and larynx, shortness of breath, tachycardia, arterial hypotension (anaphylaxis, Quincke's edema or severe shock) psychotic reactions, depression palpitations, heart failure, myocardial infarction, stroke exacerbation of bronchial asthma and bronchospasm arterial hypertension esophagitis , pancreatitis, formation of diaphragm-like narrowing of the intestine, peptic ulcer, perforation of the ulcer or bleeding from the gastrointestinal tract, melena, vomiting of blood (sometimes fatal, especially in elderly patients), ulcerative stomatitis, gastritis, exacerbation of ulcerative colitis and acute renal Crohn's disease failure, papillonecrosis (especially with long-term use), associated with an increase in urea in the blood serum and edema, liver dysfunction; the development of severe forms of skin reactions, such as bullous reactions, including erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, symptoms of aseptic meningitis: stiff neck, headache, nausea, vomiting, fever or disorientation, especially in patients with pre-existing autoimmune disorders (systemic lupus erythematosus, mixed connective tissue disease) If side effects occur, discontinue use of the drug. Contraindications: hypersensitivity to ibuprofen or other components of the drug, as well as to other non-steroidal anti-inflammatory drugs; bronchial asthma, urticaria, rhinitis, provoked by taking acetylsalicylic acid (salicylates) or other NSAIDs; ulcerative lesions of the gastrointestinal tract (peptic ulcer of the stomach and duodenum); presence history of gastrointestinal bleeding or perforation associated with NSAID therapy cerebrovascular or other acute bleeding in patients with bleeding diathesis, hematopoietic disorders of unspecified etiology in patients with severe dehydration (caused by vomiting, diarrhea or insufficient fluid intake) concomitant use of another NSAID, including specific cyclooxygenase inhibitors -2 severe renal or liver failure severe heart failure III trimester of pregnancy children under 1 year of age, children weighing less than 10 kg Drug interactions Ibufen® D Forte (as well as other drugs from the NSAID group) should not be used simultaneously with the following medications: - acetylsalicylic acid, other NSAIDs and corticosteroids: the risk of side effects from the gastrointestinal tract increases. Caution must be exercised when used simultaneously with the following drugs: - antihypertensive drugs and diuretics: since simultaneous use with NSAIDs reduces their effectiveness - antithrombotic drugs: NSAIDs can enhance the effect of drugs that reduce blood clotting - lithium, methotrexate, digoxin and phenytoin: NSAIDs can increase the concentration of these drugs in plasma (periodic monitoring of them in serum is recommended) - zidovudine: possible increase in bleeding time when used simultaneously with ibuprofen - antiplatelet drugs drugs and selective serotonin reuptake inhibitors (SSRIs): there is an increased risk of gastrointestinal bleeding - mifepristone: NSAIDs should not be used within 8-12 days after taking mifepristone, as NSAIDs may weaken its effect - tacrolimus, cyclosporine: risk of nephrotoxicity increases with simultaneous use of ibuprofen with these drugs - quinolone antibiotics: patients taking a combination of NSAIDs and quinolones may be at risk of seizures - potassium-sparing diuretics: simultaneous use with ibuprofen can lead to the development of hyperkalemia - probenecid, sulfinpyrazone: these drugs can inhibit the elimination of ibuprofen Special instructions The risk of unwanted effects can be reduced if you use the lowest effective dosage, and for the short time necessary to eliminate symptoms. Caution should be exercised when using the drug in patients: - with a history of peptic ulcer disease, especially complicated by bleeding or perforation - concomitantly taking other drugs such as corticosteroids, anticoagulants (warfarin), selective serotonin reuptake inhibitors or antiplatelet agents (acetylsalicylic acid) - with impaired liver and kidney function - with bronchial asthma, hay fever, nasal polyps or chronic obstructive respiratory disorders in history (due to an increased risk of allergic reactions, such as Quincke's edema or urticaria) - with chronic inflammatory bowel diseases (nonspecific ulcerative colitis, Crohn's disease) - with systemic lupus erythematosus and other connective tissue diseases - with uncontrolled arterial hypertension, congestive heart failure, diagnosed coronary heart disease, peripheral arterial disease and/or a history of cardiovascular disease - with cardiovascular risk factors diseases (hyperlipidemia, diabetes mellitus) - with congenital disorders of porphyrin metabolism (for example, acute intermittent porphyria) - with blood clotting disorders - immediately after a major surgical operation. Data from epidemiological and clinical studies suggest that the use of ibuprofen (especially at high doses of 2400 mg/day) and for a long time may be associated with a small increase in the risk of arterial thrombosis. You should stop using the drug if symptoms appear: skin rash, damage to the mucous membrane or other symptoms of hypersensitivity. Very rarely, severe skin reactions, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been described in connection with the use of drugs from the NSAID group. The use of the drug for chickenpox should be avoided, since the role of NSAIDs in enhancing the manifestations of this infection cannot be ruled out. Elderly patients should take the drug with caution, as the risk of side effects due to the use of NSAIDs is higher than in younger patients. Elderly patients should take the minimum effective dosage. NSAIDs may mask symptoms of infection and fever. Isolated cases of toxic amblyopia have been reported with the use of ibuprofen. With prolonged use of analgesics, headaches may occur, which should not be treated with increased doses of Ibufen D Forte. Ibufen® D Forte contains liquid maltitol, and therefore the drug should not be used in patients with rare hereditary fructose intolerance. Ibufen® D Forte contains sodium benzoate, and therefore it should be used with great caution in patients with hypersensitivity. Pregnancy and lactation period. There is no comprehensive information regarding the safety of ibuprofen in women during pregnancy. Because the effect of inhibition of prostaglandin synthesis on the human fetus remains unknown, the use of ibuprofen in the first and second trimester of pregnancy is not recommended unless absolutely necessary. The use of ibuprofen in the third trimester of pregnancy is contraindicated, since it promotes premature closure of the ductus arteriosus and can cause pulmonary hypertension in the newborn; the drug also suppresses the contractile activity of the uterus, which delays the onset of labor and prolongs labor, and also increases the risk of bleeding in the mother and child. Ibuprofen and its metabolites pass into breast milk in very low concentrations. Since there have so far been no reports of negative effects on infants, there is no need to stop breastfeeding with short-term use of the drug. Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms When taking the drug Ibufen® D Forte, dizziness may occur, which should be taken into account when driving vehicles and servicing moving mechanisms. Overdose In children, a single dose of more than 400 mg may cause overdose symptoms. In adults, the dose that can cause these symptoms has not been clearly established. The half-life during overdose ranges from 1.5 to 3 hours. Symptoms: Most patients taking clinically significant doses of NSAIDs may experience nausea, vomiting, epigastric pain, or diarrhea. You may also experience: tinnitus, headache and bleeding from the gastrointestinal tract. Severe intoxication affects the central nervous system and is manifested by drowsiness, and very rarely also agitation and disorientation or coma. Very rarely, seizures may occur. During severe intoxication, metabolic acidosis may occur and prothrombin time may increase. Possible development of acute renal failure or liver damage. Patients with bronchial asthma may experience an exacerbation of symptoms of the disease. Treatment: symptomatic and supportive treatment is used. It is necessary to monitor cardiac activity and vital signs. Oral activated charcoal should be considered within 1 hour of overdose. There is no specific antidote. Release form and packaging 100 ml or 40 ml of the drug in PET bottles with an adapter, sealed with a child-safe polyethylene screw cap with a tamper evident ring. A label is attached to each bottle. 1 bottle is placed in a cardboard pack. The packs contain approved instructions for medical use in the state and Russian languages and a syringe for oral administration. Storage conditions Store in a place protected from light at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life: 2 years Shelf life after opening the original packaging: 6 months. Do not use after expiration date. Conditions for dispensing from pharmacies Without a prescription Name and country of the manufacturing organization Medana Pharma JSC, Poland Name and country of the owner of the registration certificate Khimpharm JSC, Republic of Kazakhstan Name and country of the packaging organization Medana Pharma JSC, Poland Address of the organization accepting claims on the territory of the Republic of Kazakhstan from consumers on the quality of products (products) of Khimpharm JSC, Shymkent, Republic of Kazakhstan, st. Rashidova, 81
Contraindications
- Hypersensitivity to ibuprofen or any of the components of the drug.
- A history of hypersensitivity reactions (such as bronchospasm, rhinitis, angioedema or urticaria) after the use of ibuprofen, acetylsalicylic acid (aspirin) or other NSAIDs.
- Active gastric or duodenal ulcer/bleeding or history of relapse (two or more severe episodes of confirmed peptic ulcer or bleeding).
- A history of gastrointestinal bleeding or perforation associated with NSAID use.
- Severe heart failure, severe liver dysfunction, or severe kidney dysfunction.
- Last trimester of pregnancy.
Note!
Description of the drug Ibufen forte suspension. oral 200mg/5ml strawberry fl. 100ml on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Overdose
In childhood, overdose symptoms may occur when taking a dose of ibuprofen that exceeds 400 mg/kg. The half-life of an overdose is 1.5-3 hours.
Symptoms The majority of patients receiving clinically significant amounts of the drug developed only nausea, vomiting, epigastric pain, and very rarely diarrhea. Tinnitus, headache, and gastrointestinal bleeding may also occur. With more severe poisoning, toxic damage to the central nervous system is possible, which manifests itself as vertigo, drowsiness, and sometimes agitation and disorientation or coma. Sometimes patients experience seizures
Treatment. Treatment should be symptomatic and supportive and include maintaining the airway and monitoring cardiac and vital signs until the condition returns to normal. It is recommended to administer activated charcoal orally or gastric lavage within 1 hour after administering a potentially toxic dose of the drug.