Description of the drug IBUFEN FORTE
With simultaneous use, ibuprofen reduces the effect of antihypertensive drugs (ACE inhibitors, beta-blockers), diuretics (furosemide, hydrochlorothiazide).
When used simultaneously with anticoagulants, their effect may be enhanced.
When used simultaneously with GCS, the risk of side effects from the gastrointestinal tract increases.
When used simultaneously, ibuprofen can displace indirect anticoagulants (acenocoumarol), hydantoin derivatives (phenytoin), and oral hypoglycemic drugs, sulfonylurea derivatives, from compounds with blood plasma proteins.
When used simultaneously with amlodipine, a slight decrease in the antihypertensive effect of amlodipine is possible; with acetylsalicylic acid - the concentration of ibuprofen in the blood plasma decreases; with baclofen - a case of increased toxic effects of baclofen has been described.
When used simultaneously with warfarin, an increase in bleeding time is possible; microhematuria and hematomas were also observed; with captopril - the antihypertensive effect of captopril may be reduced; with cholestyramine - a moderate decrease in the absorption of ibuprofen.
When used simultaneously with lithium carbonate, the concentration of lithium in the blood plasma increases.
When used simultaneously with magnesium hydroxide, the initial absorption of ibuprofen increases; with methotrexate - the toxicity of methotrexate increases.
The simultaneous use of NSAIDs and cardiac glycosides can lead to worsening heart failure, a decrease in glomerular filtration rate and an increase in the concentration of cardiac glycosides in the blood plasma.
There is evidence of the likelihood of an increase in the concentration of methotrexate in the blood plasma during the use of NSAIDs.
With simultaneous use of NSAIDs and cyclosporine, the risk of nephrotoxicity increases.
NSAIDs may reduce the effectiveness of mifepristone, so taking NSAIDs should be started no earlier than 8-12 days after stopping mifepristone.
Concomitant use of NSAIDs and tacrolimus may increase the risk of nephrotoxicity.
Concomitant use of NSAIDs and zidovudine may lead to increased hematotoxicity. There is evidence of an increased risk of hemarthrosis and hematomas in HIV-positive patients with hemophilia who received concomitant treatment with zidovudine and ibuprofen.
In patients receiving concomitant treatment with NSAIDs and quinolone antibiotics, the risk of seizures may increase.
In patients receiving concomitant NSAIDs and myelotoxic drugs, hematotoxicity increases.
With the simultaneous use of ibuprofen and cefamandole, cefoperazone, cefotetan, valproic acid, plicamycin, the incidence of hypoprothrombinemia increases.
With the simultaneous use of ibuprofen and drugs that block tubular secretion, there is a decrease in excretion and an increase in plasma concentration of ibuprofen.
With the simultaneous use of ibuprofen and inducers of microsomal oxidation (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants), there is an increase in the production of hydroxylated active metabolites and an increased risk of developing severe intoxications.
Ibuprofen FT
Ibuprofen FT suspension is designed specifically for children. For oral administration.
Patients with stomach hypersensitivity are advised to take ibuprofen with meals.
For short term use only. The lowest effective dose should be used for the shortest possible period of time.
Read the instructions carefully before taking ibuprofen.
Shake the bottle thoroughly before use.
Fever (fever) and pain
The dosage regimen for children depends on the age and body weight of the child. The maximum daily dose should not exceed 30 mg/kg of the child’s body weight with intervals between doses of the suspension of 6-8 hours.
Dosage regimen of the drug at a dosage of 100 mg/5 ml
5 ml of suspension contains 100 mg of ibuprofen, respectively, 1 ml of suspension contains 20 mg of ibuprofen.
Children aged 3-6 months (child's body weight from 5 to 7.6 kg): 2.5 ml (50 mg) up to 3 times within 24 hours, no more than 7.5 ml (150 mg) per day.
Children aged 6-12 months (child's body weight from 7.7 to 9 kg): 2.5 ml (50 mg) up to 3-4 times within 24 hours, no more than 10 ml (200 mg) per day.
Children aged 1-3 years (child's body weight from 10 to 16 kg): 5 ml (100 mg) up to 3 times within 24 hours, no more than 15 ml (300 mg) per day.
Children aged 4-6 years (child weight from 17 to 20 kg): 7.5 ml (150 mg) up to 3 times within 24 hours, no more than 22.5 ml (450 mg) per day.
Children aged 7-9 years (child weight from 21 to 30 kg): 10 ml (200 mg) up to 3 times within 24 hours, no more than 30 ml (600 mg) per day.
Children aged 10-12 years (child's body weight from 31 to 40 kg): 15 ml (300 mg) up to 3 times within 24 hours, no more than 45 ml (900 mg) per day.
The duration of treatment is no more than 3 days.
Do not exceed the indicated dose.
If, when taking the suspension within 24 hours (in children aged 3-5 months) or within 3 days (in children aged 6 months and older), symptoms persist or worsen, you should stop treatment and consult a doctor.
Post-immunization fever:
Children aged 3 months and older (body weight 5 kg or more): 2.5 ml (50 mg) suspension once. If necessary, an additional 2.5 ml (50 mg) after 6 hours. Do not use more than 5 ml (100 mg) within 24 hours.
If the elevated body temperature does not decrease, consult a doctor.
Dosage regimen of the drug in date 200 mg/5 ml
5 ml of suspension contains 200 mg of ibuprofen, respectively, 1 ml of suspension contains 40 mg of ibuprofen.
Children aged 1-3 years (child's body weight from 10 to 16 kg): 2.5 ml (100 mg) up to 3 times within 24 hours, no more than 7.5 ml (300 mg) per day.
Children aged 4-6 years (child's body weight from 17 to 20 kg): 3.75 ml (150 mg) up to 3 times within 24 hours, no more than 11.25 ml (450 mg) per day.
Children aged 7-9 years (child weight from 21 to 30 kg): 5 ml (200 mg) up to 3 times within 24 hours, no more than 15 ml (600 mg) per day.
Children aged 10-12 years (child's body weight from 31 to 40 kg): 7.5 ml (300 mg) up to 3 times within 24 hours, no more than 22.5 ml (900 mg) per day.
Children aged 13 years and older (body weight more than 40 kg): 7.5-10 ml (300-400 mg) up to 3 times within 24 hours, no more than 30 ml (1200 mg) per day.
The duration of treatment is no more than 3 days. Do not exceed the indicated dose. If symptoms persist or worsen, stop treatment and consult a doctor.
Use in patients with renal failure
The use of the drug in patients with severe renal failure is contraindicated (see also section “Contraindications”).
Use in patients with liver failure
The use of the drug in patients with severe liver failure is contraindicated (see also section “Contraindications”),
Mode of application
The dosing syringe with insert included in the package allows you to accurately dose the drug.
1. Remove the cap from the bottle. Insert, if necessary, the insert into the neck of the bottle so that it fits tightly. Insert the dispenser syringe into the hole in the insert (the diagram is shown below).
2. Turn the bottle with the syringe upside down and smoothly pull the syringe plunger down, drawing the suspension into the syringe to the desired mark (the diagram is shown below).
3. Return the bottle with the syringe to its original vertical position. Make sure the syringe plunger has not moved and the plunger tip is still level with the desired mark. Pull the syringe out of the insert hole (the insert should be left fixed in the neck of the bottle). Close the bottle with a cap.
4. The patient should assume an upright position. Slowly press down on the plunger of the syringe, gradually releasing the medication. Sharp pressure on the piston is not allowed. When releasing the drug, the syringe opening should be directed towards the inner surface of the cheek to prevent the patient from choking.
5. Disassemble the syringe and rinse it in clean drinking water. Allow the barrel and plunger of the syringe to dry naturally. Keep the dispenser syringe out of the reach of children.
Ibufen ultra capsules 200 mg N10
IBUFEN ultra International nonproprietary name of the drug Ibuprofen
Composition Each capsule contains: active ingredient: ibuprofen 200 mg excipients: macrogol 600 (E1521), potassium hydroxide (E525), purified water. Gelatin capsule: liquid maltitol (E965), liquid sorbitol, non-crystallizing (E420), gelatin (E441), patent blue dye (E131), purified water.
Description Oval-shaped gelatin capsules with a translucent blue shell containing a viscous liquid.
Dosage form Soft gelatin capsules
Pharmacotherapeutic group Nonsteroidal anti-inflammatory and antirheumatic drugs, propionic acid derivatives. ATS code: M 01 AE 01
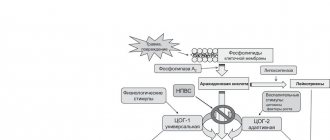
Pharmacological properties Ibuprofen is a derivative of propionic acid. It has analgesic, antipyretic and anti-inflammatory effects. The mechanism of action of ibuprofen is primarily due to the suppression of prostaglandin biosynthesis by reducing the activity of cyclooxygenase (COX), an enzyme that regulates the conversion of arachidonic acid into prostaglandins, prostacyclin and thromboxane. At the same time, as a result of irreversible inhibition of the cyclooxygenase pathway of arachidonic acid metabolism, the formation of prostaglandins decreases. A decrease in the concentration of prostaglandins at the site of inflammation is accompanied by a decrease in the formation of bradykinin, endogenous pyrogens, other biologically active substances, oxygen radicals and NO. All this leads to a decrease in the activity of the inflammatory process (anti-inflammatory effect of ibuprofen) and is accompanied by a decrease in pain reception (analgesic effect). A decrease in the concentration of prostaglandins in the cerebrospinal fluid leads to normalization of body temperature (antipyretic effect). Ibufen ultra soft gelatin capsules contain ibuprofen in liquid form. Gelatin capsules ensure high dosing accuracy of the substances placed in them. The capsule shell protects the active substance from light, air and moisture, and also eliminates the unpleasant taste and smell of the medicinal substance when taken. The capsule disintegrates in the gastrointestinal tract faster than dragees and tablets, and its liquid contents are absorbed faster and more easily by the human body, ensuring high bioavailability of ibuprofen. After oral administration, more than 80% of ibuprofen is absorbed from the digestive tract. 90% of the drug binds to blood plasma proteins (mainly albumin). The period to achieve maximum concentration in blood plasma when taken on an empty stomach is 45 minutes, when taken after meals - 1.5-2.5 hours; in synovial fluid - 2-3 hours, where higher concentrations are created than in blood plasma. The drug does not accumulate in the body. Ibuprofen is metabolized mainly in the liver. Subject to presystemic and postsystemic metabolism. After absorption, about 60% of the pharmacologically inactive R form of ibuprofen is slowly transformed into the active S form. 60-90% of the drug is excreted by the kidneys in the form of metabolites and products of their combination with glucuronic acid, to a lesser extent, with bile and no more than 1% is excreted unchanged. After taking a single dose, the drug is completely eliminated within 24 hours.
Indications for use Increased body temperature of various origins with: - colds, - acute respiratory viral infections, - influenza; - sore throat (pharyngitis); - childhood infections accompanied by increased body temperature; - post-vaccination reactions. Pain syndrome of various origins of weak and moderate intensity with: - ear pain due to inflammation of the middle ear; - toothache, painful teething; - headache, migraine; - painful menstruation, - neuralgia, - rheumatic pain, - muscle and joint pain, - musculoskeletal injuries.
Contraindications The drug Ibufen Ultra should not be used in case of individual hypersensitivity to any component of the drug, as well as to other non-steroidal anti-inflammatory drugs; - ever-previous manifestations of allergy symptoms in the form of a runny nose, skin rashes or bronchospastic reactions after using acetylsalicylic acid or other non-steroidal anti-inflammatory drugs; - peptic ulcer of the stomach and duodenum in the acute phase, - severe insufficiency of liver and kidney function; — blood diseases: hemophilia, hypocoagulation, hemorrhagic diathesis; - in the third trimester of pregnancy.
Interaction with other drugs Ibuprofen (as well as other drugs from the group of non-steroidal anti-inflammatory drugs) should not be used simultaneously with the following drugs: - acetylsalicylic acid or other non-steroidal anti-inflammatory drugs - the risk of side effects from the gastrointestinal tract increases, - antihypertensive drugs drugs and diuretics - drugs from the group of non-steroidal anti-inflammatory drugs can cause a decrease in the effectiveness of these drugs, - anticoagulants - limited clinical data indicate that non-steroidal anti-inflammatory drugs can enhance the effect of drugs that reduce blood clotting, - lithium and methotrexate - it has been proven that non-steroidal anti-inflammatory drugs anti-inflammatory drugs can cause an increase in plasma concentrations of both lithium and methotrexate, - zidovudine - there is evidence of an increase in bleeding time in patients taking ibuprofen and zidovudine at the same time, - mineralocorticoids, glucocorticoids - increased side effects, - sulfonylurea derivatives - increased hypoglycemic effect, - antacids and cholestyramine reduce absorption, - caffeine enhances the analgesic effect.
Special instructions Before taking Ibufen ultra, you should consult a doctor if the following have been previously confirmed: - bronchial asthma, urticaria, - liver and kidney diseases, - a history of gastric and duodenal ulcers - arterial hypertension. During long-term treatment, monitoring of the peripheral blood picture and the functional state of the liver and kidneys is necessary. When symptoms of gastropathy appear, careful monitoring is indicated, including esophagogastroduodenoscopy, a complete blood count (hemoglobin determination), and a stool test for occult blood. If it is necessary to determine 17-ketosteroids, the drug should be discontinued 48 hours before the study.
Pregnancy and lactation The use of ibuprofen in the first 6 months of pregnancy requires caution and is permitted only as prescribed by a doctor, after assessing the expected benefits and possible risks. Ibuprofen should not be used in the last trimester of pregnancy. Ibuprofen may be excreted in breast milk in small quantities. There are no known cases of side effects in infants, however, it is recommended to stop breastfeeding during treatment with ibuprofen.
Impact on the ability to drive vehicles and maintain mechanical equipment There is no information on contraindications to driving and servicing machinery while taking Ibufen ultra.
Method of administration and dosage For oral use. 1 capsule contains 200 mg of ibuprofen. Adults and children over 12 years of age (over 40 kg): single dose 200-400 mg (1-2 capsules). Then, if necessary, 1-2 capsules every 4-6 hours. The maximum daily dose is 6 capsules (1200 mg ibuprofen). The minimum interval between subsequent doses is 4-6 hours. The capsule must be swallowed whole with a small amount of water. Capsules should not be cracked, sucked or chewed. When using the drug in children, the child’s weight should be taken into account for accurate dosing. The drug should not be used for more than 5 days as an analgesic and for more than 3 days as an antipyretic without medical supervision.
Overdose If you take a higher dose of the drug than recommended, you should immediately contact your doctor or pharmacist. Symptoms of overdose: abdominal pain, vomiting, lethargy, headache, tinnitus, depression, drowsiness, metabolic acidosis, coma, hemorrhagic diathesis, decreased blood pressure, convulsions, acute renal failure, liver dysfunction, tachycardia, bradycardia. High doses of ibuprofen are usually well tolerated, provided that no other drugs are used at the same time. Measures in case of overdose: gastric lavage (only within an hour after taking the drug), activated carbon, alkaline drinking, symptomatic therapy (correction of the acid-base state, blood pressure).
Side effects Side effects are classified by frequency using the following definitions: - very common: > 1/10 - common: > 1/100 to < 1/10 - uncommon: > 1/1000 to < 1/100 - rare: > 1 /10,000 to <1/1000 - very rare: <1/10,000 When using Ibufen ultra for 2-3 days, practically no side effects are observed. In case of long-term use, the following side effects may occur: From the gastrointestinal tract Uncommon: nausea, heartburn, diarrhea, abdominal pain. Rarely: vomiting, flatulence, constipation, inflammation of the gastrointestinal tract. Very rare: ulceration of the gastrointestinal mucosa, bleeding, Crohn's disease, impaired liver function. From the nervous system: Uncommon: headache; Rare: dizziness, agitation, insomnia, drowsiness. In isolated cases, hearing loss, tinnitus, and depression have been described. From the urinary system Rarely: edematous syndrome, Very rarely: acute renal failure, allergic nephritis, polyuria, cystitis. From the hematopoietic organs Very rare: anemia (including hemolytic, aplastic), thrombocytopenia and thrombocytopenic purpura, agranulocytosis, leukopenia. Allergic reactions Uncommon: skin rash, itching, urticaria, Very rare: Quincke's edema, angioedema, anaphylactoid reactions, anaphylactic shock, bronchospasm, fever, erythema multiforme exudative (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome ), eosinophilia, allergic rhinitis. From the cardiovascular system: Heart failure, increased blood pressure, tachycardia. Any side (unusual) effects, including those not listed in the package insert, should be reported to your doctor.
Shelf life: 2 years Do not use after the expiration date indicated on the package. The medicine should be stored out of the reach of children.
Storage conditions Store in original packaging at a temperature not exceeding 25C.
Packaging Ibufen ultra, soft gelatin capsules 200 mg: 10 capsules each in a blister made of PVC/PVDC and aluminum foil. 10 (1 blister) or 20 (2 blisters) capsules together with an insert in a cardboard box.