Infibeta: indications and instructions for use
- Infibeta lyophilisate is a human protein, which is the reaction of the biosystem to the penetration of a viral foreign agent into it. It slows down the protein reproduction process of viruses.
- By enhancing the performance of immune response regulators, the drug thereby reduces the impact of protective macrophages on the electrical insulating sheath of nerve fibers.
- Indicated for therapeutic effects in the development of multiple sclerosis.
- Infibet contains human interferon-beta and albumin, which prevent the development of viral replication.
- How to take Infibeta is determined by a consultation of a neurologist, immunologist and other medical specialists. The powder is combined with a special mixer and administered as injections.
- Infibeta during pregnancy, lactation productivity and hypersensitivity of the protective barrier is not prescribed for consumption.
- Infibeta is prohibited for use by children under 18 years of age.
Infibeta®
Immune disorders
The use of cytokines in patients with monoclonal gammopathy was sometimes accompanied by a systemic increase in capillary permeability with the development of shock and death.
Gastrointestinal disorders
In rare cases, while using the drug Infibeta®, the development of pancreatitis was observed, in most cases associated with the presence of hypertriglyceridemia.
Nervous system diseases
Caution should be exercised when prescribing Infibeta® to patients with current or history of depressive disorders, especially to patients with a history of suicidal thoughts. Depression and suicidal ideation are more common in patients with multiple sclerosis than in the general population and are associated with interferon use.
Patients should be informed that if depression or suicidal ideation occurs, they should report it to their healthcare provider immediately. Patients with manifestations of depression should be closely monitored, and therapy with Infibeta® should be adjusted accordingly. The need for continued treatment with Infibeta® should be reconsidered. Infibeta® should be used with caution in patients with a history of seizures and in patients taking anticonvulsants, especially if epilepsy cannot be controlled with these drugs (see sections “Side effects”, “Interaction with other drugs”).
This medicine contains human albumin and as a result there is a very low risk of transmitting viral infections. The risk of transmission of Creutzfeldt-Jakob disease cannot be excluded.
Changes in laboratory parameters
Patients with thyroid dysfunction are advised to have their thyroid function checked regularly and otherwise as clinically indicated.
In addition to standard laboratory tests prescribed for the management of patients with multiple sclerosis, before starting therapy with Infibeta®, as well as regularly during treatment, it is recommended to conduct a detailed blood test, including determining the leukocyte formula, platelet count and biochemical analysis, as well as checking liver function (eg, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyltransferase (γ-GT) activities).
When managing patients with anemia, thrombocytopenia, leukopenia (individually or in combination), more careful monitoring of a complete blood count may be required, including determination of the number of red blood cells, white blood cells, platelets and leukocyte formula.
Patients with neutropenia should be closely monitored for fever or infection. There are reports of cases of thrombocytopenia with a marked decrease in platelet count.
Liver and biliary tract dysfunctions
Clinical studies have shown that interferon beta-1b therapy can often lead to an asymptomatic increase in the activity of liver transaminases, which in most cases is mild and transient.
As with other beta interferons, severe liver damage (including liver failure) is rare with Infibeta®. The most severe cases have been observed in patients exposed to hepatotoxic drugs or substances, as well as in certain concomitant diseases (eg, metastatic malignancies, severe infections and sepsis, alcoholism).
When treating with Infibeta®, it is necessary to monitor liver function (including assessment of the clinical picture).
Increased transaminase activity in the blood serum requires careful monitoring and examination. If there is a significant increase in transaminase activity in the blood serum or signs of liver damage (for example, jaundice) appear, the drug should be discontinued.
In the absence of clinical signs of liver damage or after normalization of the activity of liver enzymes, it is possible to resume therapy with Infibeta® with monitoring of liver function.
Renal and urinary tract dysfunction
Infibeta should be used with caution in patients with severe renal impairment under close monitoring.
Nephrotic syndrome
There have been case reports of the occurrence of nephrotic syndrome with various nephropathies, including focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), membranoproliferative glomerulonephritis (MPGN) and membranous nephropathy (MN), when treated with drugs containing interferon beta.
The onset of the disease was noted both at various periods during treatment and several years after interferon beta therapy. It is recommended to regularly monitor for early symptoms such as edema, proteinuria and renal dysfunction, especially in patients at high risk of developing kidney disease. Treatment for nephrotic syndrome should be initiated early, and the need for continued treatment with interferon beta-1b should be reconsidered.
Diseases of the cardiovascular system
Infibeta® should be used with caution in patients with heart disease. Patients with severe heart disease, in particular with clinically significant chronic heart failure with clear symptoms of fluid stagnation, coronary heart disease or arrhythmia, should be under the supervision of a physician for timely detection of possible deterioration of the condition, especially at the beginning of treatment.
Although there is no evidence of a direct cardiotoxic effect of interferon beta-1b, the flu-like symptoms that commonly occur with the administration of interferon betas may be a precipitating factor in patients with severe cardiac disease. There are rare reports of cases of cardiomyopathy.
If cardiomyopathy develops during treatment with Infibeta® and it is suspected that this is related to the use of the drug, then treatment with Infibeta® should be discontinued.
Thrombotic microangiopathy (TMAP)
During treatment with interferon beta drugs, cases of thrombotic microangiopathy, manifested as thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS), including death, have been reported.
These complications have been reported at various times during treatment and may appear several weeks or several years after the start of interferon beta treatment. Early clinical symptoms of the disease include thrombocytopenia, sudden onset of hypertension, fever, signs of central nervous system disorders (eg, confusion, paresis), and renal dysfunction.
Laboratory findings, such as a decrease in platelet count, an increase in plasma lactate dehydrogenase (LDH) levels, suggest the occurrence of thrombotic microangiopathy (TMAP) due to activation of hemolysis and the detection of schizocytes (red blood cell fragments) in the blood smear. If clinical signs of TMAP occur, continued testing of platelet levels, plasma lactate dehydrogenase (LDH), blood smears, and renal function should be performed. If TMAP is confirmed, immediate treatment (including plasmapheresis) must be carried out, and treatment with Infibeta® must be discontinued.
General disorders and injection site disorders
Serious allergic reactions (rare but acute and severe, such as bronchospasm, anaphylaxis and urticaria) may occur. If a severe reaction occurs, Infibeta® should be discontinued and appropriate treatment should be prescribed.
Cases of necrosis at the injection site have been observed in patients receiving Infibeta® (see section "Side effects").
Necrosis can be extensive and extend into muscle fascia as well as fatty tissue and, as a result, lead to scar formation. In some cases, removal of dead areas or, less commonly, skin grafting is necessary. The healing process can take up to 6 months.
If there are signs of damage to the integrity of the skin (for example, leakage of fluid from the injection site), the patient should consult a doctor before continuing with Infibeta® injections.
If multiple foci of necrosis appear, treatment with Infibeta® should be stopped until the damaged areas are completely healed. In the presence of one lesion, if the necrosis is not too extensive, the use of the drug Infibeta® can be continued, since in some patients the healing of the necrotic area at the injection site occurred during the use of the drug Infibeta®.
In order to reduce the risk of reaction and necrosis at the injection site, patients should be advised to:
- carry out injections strictly following the rules of asepsis;
- change the injection site each time;
- administer the drug strictly subcutaneously.
The use of an auto-injector reduces the incidence of injection site reactions. You should periodically monitor the correctness of self-injections, especially if local reactions occur.
Immunogenicity
As with any drug containing proteins, there is a possibility of antibody formation when using Infibeta®. In a number of controlled clinical studies, serum samples were tested every 3 months to detect the formation of antibodies to interferon beta-1b.
In these studies, it was shown that neutralizing antibodies to interferon beta-1b developed in 23-41% of patients, which was confirmed by at least two subsequent positive laboratory test results. In 43-55% of these patients, subsequent laboratory tests revealed a stable absence of antibodies to interferon beta-1b.
In these studies, the emergence of neutralizing activity was associated with a decrease in clinical efficacy only in terms of relapse rate. The results of some analyzes suggest a greater severity of this effect in patients with higher titers of neutralizing antibodies.
In a study of patients with clinically isolated syndrome suggestive of multiple sclerosis, neutralizing activity, measured every 6 months, was detected at least once in 32% (89) of treated patients. Based on laboratory testing, 60% (53) of these patients were consistently negative for interferon beta-1b antibodies. In this study, the development of neutralizing activity was associated with a significant increase in the number of newly detected lesions and an increase in lesion volume on T2-weighted magnetic resonance images. It is unlikely that the development of neutralizing activity is associated with a decrease in clinical efficacy relative to the time to the onset of clinically definite multiple sclerosis or progression on the EDSS scale.
The development of neutralizing activity was not associated with the occurrence of any adverse reactions. The decision to continue or discontinue therapy should be based on clinical disease activity rather than neutralizing activity status.
Use in children
There have been no systematic studies of the effectiveness and safety of Infibeta in children and adolescents under 18 years of age.
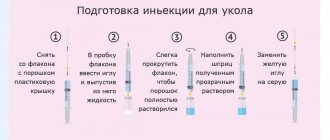
Rules for preparing an injection solution and performing subcutaneous injections
To prepare a solution for subcutaneous injection, mix the contents of the bottles with lyophilisate and solvent. To do this you need to prepare:
1 bottle of lyophilisate bottle of solvent*
1 syringe with a capacity of 2 ml
1 syringe with a capacity of 1 ml**
2 long needles
1 short needle
2 alcohol wipes.
Do not use a bottle with defects in the glass or closure system.
Attention
:
*The bottle with the solvent should be removed from the refrigerator 10-15 minutes before the injection.
**If you plan to use an auto-injector, use a 1 ml syringe to withdraw the injection solution.
— Wash your hands with warm water and soap.
— Remove the plastic covers from the bottles.
— Treat the rubber plugs with an alcohol wipe.
— Remove the 2 ml syringe from the blister pack.
— Place the long needle on the syringe and remove the protective cap.
— Pierce the rubber stopper of the solvent bottle with a long needle and remove 1.2 ml of solution from the bottle.
— Remove the filled syringe along with the needle from the bottle, remove air bubbles from the syringe, change the needle to a second long one.
— Pierce the rubber stopper of the bottle with lyophilisate, pointing the needle towards the side wall of the bottle. Slowly inject the solvent (1.2 ml) down the side of the vial, avoiding foaming.
- Gently rock the bottle to dissolve its contents without removing the syringe from the bottle. Do not shake! The finished solution must be transparent and free of visible particles.
— Turn the bottle over and draw the prepared solution into the syringe, making sure that the end of the needle is immersed in the solution. At the beginning of treatment, the volume of solution indicated in the dose titration table is drawn into the syringe.
— If an auto-injector is used, the solution is taken with a 1 ml syringe.
- Remove the syringe from the bottle, release air and excess drug from the syringe to the 1.0 ml mark, holding the syringe vertically, with the needle up.
— Replace the long needle with a short one for a subcutaneous injection.
Recommendations for choosing the site of subcutaneous injections
The drug is injected with a syringe with a short needle subcutaneously into soft tissues located away from the joints and nerves. Injection sites should be located in the following parts of the body:
- Arms (back of the shoulder).
- Abdomen (excluding the navel and waist area).
- Buttocks.
— Hips (front and outer lateral surfaces, excluding the groin and knee areas).
Injection sites must be alternated, choosing a new one each time, according to the diagram in Figure 1. For convenience, write down where and when the injections were made or make appropriate notes in the table in Figure 1.
Attention!
Do not inject into areas where there are bumps, swelling, hard knots, or pain. Do not inject into areas of skin that are discolored, or if there are crusts, depressions, or lesions. If you notice such changes in yourself, consult your doctor.
Rules for performing a subcutaneous injection without an auto-injector
:
- Wipe the skin at the injection site with an alcohol wipe and wait until the skin dries.
- Take a filled syringe (2 ml capacity) and remove the protective cap from the needle (short).
- Gather the skin into a fold.
— With a confident movement, insert the needle into the raised area of skin at an angle of 90° along its entire length.
- Slowly inject the entire solution contained in the syringe (no more than 1.0 ml), pressing evenly on the plunger, and remove the needle from the skin.
— Press an alcohol pad to the injection site and lightly massage the skin.
— Dispose of used syringes, needles, vials and wipes into a waste container.
Rules for performing subcutaneous injection using an auto-injector
:
- Wipe the skin at the injection site with an alcohol wipe and wait until the skin dries.
— Take a filled 1 ml syringe with a short needle and remove the protective cap from the needle.
— Place the filled syringe into the auto-injector and give yourself a subcutaneous injection, following the rules described in the instructions for the auto-injector.
The solution for subcutaneous injection is prepared immediately before use.