Clinical manifestations of neurological complications of diabetes mellitus (DM) are varied and similar to the symptoms of other diseases, which often leads to late initiation of pathogenetic therapy. Normoglycemia is the main condition for preventing diabetic polyneuropathy (DP); achieving it is not always possible; the disease, as a rule, has a progressive course. Diagnosis D.P. is established on the basis of a clinical examination, but the absence of symptoms of the disease does not exclude its presence and requires instrumental examination [1].
DP is observed in 30-50% of patients with diabetes and has various variants. Its frequency is related to the duration of the disease, the effectiveness of glycemic control and other risk factors. The most common form of DP is chronic distal symmetric sensory or sensorimotor DP (DSDP), which develops in a third of patients with diabetes. DDSP is accompanied by neuropathic pain syndrome and leads to a decrease in the quality of life of patients [2].
Treatment of patients with DDSP includes reducing the severity of pain (symptomatic therapy) and restoring the structure of the affected nerves (pathogenetic therapy). Treatment should be comprehensive, including drugs that affect different aspects of the development of the pathological process. Neurotropic vitamins and metabolic drugs affect a number of biochemical processes occurring in nervous tissue [3, 4]. One such drug is cocarnit, which contains triphosadenine, cocarboxylase, cyanocobalamin and nicotinamide. Each of these components performs a specific function in metabolic processes. A number of clinical studies have demonstrated the effectiveness and safety of cocarnit in the treatment of patients with DDSP [5].
The purpose of the study was to study the effect of cocarnit on the functions of peripheral nerves in patients with DDSP.
Cocarnit (liof.d/sol. i.m. 187.125 amp. 2ml No. 3+sol.)
A country
Egypt
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Compound
1 ampoule contains: triphosadenine disodium trihydrate 10 mg, cocarboxylase 50 mg, cyanocobalamin 0.5 mg, nicotinamide 20 mg.
Excipients: glycine - 105.875 mg, methyl parahydroxybenzoate - 0.6 mg, propyl parahydroxybenzoate - 0.15 mg. Solvent: lidocaine hydrochloride - 10 mg, water for injection - up to 2 ml. Lyophilisate for preparing a solution for intramuscular administration in the form of a pink lyophilized mass. The reconstituted solution is transparent and pink in color.
pharmachologic effect
The drug is a rationally selected complex of metabolic substances and vitamins. Trifosadenine is an adenosine derivative that stimulates metabolic processes. Has a vasodilating effect, incl. to the coronary and cerebral arteries. Improves metabolism and energy supply to tissues. Has hypotensive and antiarrhythmic effects. Under the influence of ATP, blood pressure decreases, smooth muscles relax, and the conduction of nerve impulses improves. Cocarboxylase is a coenzyme formed in the body from thiamine (vitamin B1) supplied from outside. It is part of the carboxylase enzyme, which catalyzes the carboxylation and decarboxylation of β-keto acids. Indirectly promotes the synthesis of nucleic acids, proteins and lipids. Reduces the concentration of lactic and pyruvic acids in the body and promotes the absorption of glucose. Improves trophism of nervous tissue. Cyanocobalamin (vitamin B12) is converted into methylcobalamin and 5-deoxyadenosylcobalamin in the body. Methylcobalamin is involved in the conversion of homocysteine to methionine and S-adenosylmethionine - key reactions in the metabolism of pyrimidine and purine bases (hence DNA and RNA). If there is insufficiency of the vitamin in this reaction, it can be replaced by methyltetrahydrofolic acid, and folate-requiring metabolic reactions are disrupted. 5-deoxyadenosylcobalamin serves as a cofactor in the isomerization of L-methylmalonyl-CoA to succinyl-CoA, an important reaction in carbohydrate and lipid metabolism. Vitamin B12 deficiency leads to impaired proliferation of rapidly dividing cells of hematopoietic tissue and epithelium, as well as impaired formation of the myelin sheath of neurons. Nicotinamide is one of the forms of vitamin PP, participates in redox processes in the cell, improves carbohydrate and nitrogen metabolism, and regulates tissue respiration.
Indications for use
Symptomatic treatment of diabetic polyneuropathy.
Mode of application
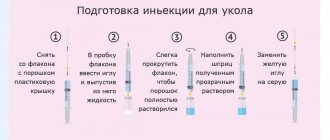
The drug is injected deep IM (into the gluteal muscle). In cases of severe pain, it is advisable to start treatment with intramuscular administration of 2 ml (1 amp.)/day until acute symptoms subside. Duration of use: 9 days. After improvement of symptoms or in cases of moderately severe symptoms of polyneuropathy, 2 ml (1 amp.) is prescribed 2-3 times a week for 2-3 weeks. The recommended course of treatment is 3-9 injections depending on the severity of the disease. The duration of treatment and repeated courses are determined by the doctor depending on the nature and severity of the disease.
Interaction
In patients using hypoglycemic agents of the biguanide group (metformin), due to impaired absorption of cyanocobalamin from the gastrointestinal tract, a decrease in the concentration of cyanocobalamin in the blood may be observed. Drug interactions with other hypoglycemic agents have not been described. Cyanocobalamin is not compatible with ascorbic acid, heavy metal salts, thiamine, thiamine bromide, pyridoxine, riboflavin, folic acid. Cyanocobalamin should not be used simultaneously with drugs that increase blood clotting. In addition, concomitant use of cyanocobalamin with chloramphenicol should be avoided. Aminoglycosides, salicylates, antiepileptic drugs, colchicine, potassium preparations reduce the absorption of cyanocobalamin. When combined with drugs containing triphosadenine and dipyridamole, the effect of dipyridamole is enhanced, in particular its vasodilating effect. Dimiridamol enhances the effect of triphosadenine. Some antagonism appears when the drug is used together with purine derivatives (caffeine, theophylline). It should not be administered simultaneously with cardiac glycosides in large doses, as the risk of adverse reactions from the cardiovascular system increases. When used simultaneously with xanthinol nicotinate, the effect of the drug is reduced. Nicotinamide potentiates the effect of sedatives, tranquilizers, and antihypertensive drugs.
Side effect
The frequency of adverse reactions is given in accordance with the WHO classification: very often (more than 1/10); often (less than 1/10, but more than 1/100); uncommon (less than 1/100, but more than 1/1000); rare (less than 1/1000, but more than 1/10,000); very rare (less than 1/10,000), including isolated cases; frequency unknown. From the immune system: rarely - allergic reactions (skin rash, difficulty breathing, anaphylactic shock, Quincke's edema). From the nervous system: very rarely - dizziness, headache, agitation, confusion. From the cardiovascular system: - very rarely - tachycardia; - in some cases, bradycardia, arrhythmia; — frequency unknown — pain in the heart area, redness of the skin of the face and upper half of the body with a tingling and burning sensation, “hot flashes.” From the gastrointestinal tract: very rarely - vomiting, diarrhea. From the skin and subcutaneous tissues: very rarely - increased sweating, acne, itching, urticaria. From the side of musculoskeletal and connective tissue: very rarely - convulsions. General disorders and disorders at the injection site: very rarely - irritation, pain and burning at the injection site, weakness may occur. If any of these adverse reactions worsen or any other adverse reactions not listed in the instructions appear, the patient should inform the doctor. If severe adverse reactions develop, the drug is discontinued.
Contraindications
- hypersensitivity to any component of the drug or solvent; - cardiovascular diseases (acute heart failure, acute myocardial infarction, uncontrolled arterial hypertension, arterial hypotension, severe forms of bradyarrhythmias, AV block II-III degree, chronic heart failure (III-IV functional class according to NYHA), cardiogenic shock and others types of shock, QT prolongation syndrome, thromboembolism, hemorrhagic stroke); - inflammatory lung diseases, COPD, bronchial asthma; - pregnancy; - period of breastfeeding; — age up to 18 years; - hypercoagulation (including in acute thrombosis), erythremia, erythrocytosis; - peptic ulcer of the stomach or duodenum in the acute phase; - gout; - hepatitis, cirrhosis of the liver. The drug should be used with caution for angina pectoris.
Overdose
The components of the drug Cocarnit have a wide therapeutic range. Symptoms Trifosadenine. Exceeding the maximum daily dose (about 600 mg for an adult) can lead to the development of the following symptoms: dizziness, decreased blood pressure, short-term loss of consciousness, arrhythmia, AV block of the second and third degrees, asystole, bronchospasm, ventricular disorders, sinus bradycardia and tachycardia. Cocarboxylase. The following symptoms have been reported after administration of more than 100 times the recommended dose: headache, muscle spasm, muscle weakness, paralysis, arrhythmia. Cyanocobalamin. After parenteral administration of a high dose, eczematous skin disorders and benign acne were observed. When used in high doses, hypercoagulation and disruption of purine metabolism may develop. Nicotinamide. When used in large doses, hyperpigmentation, jaundice, amblyopia, weakness, and exacerbation of gastric and duodenal ulcers were observed. With long-term use, the development of steatohepatosis, an increase in the concentration of uric acid in the blood, and impaired glucose tolerance were noted. Treatment Administration of the drug is stopped immediately, symptomatic therapy is prescribed, incl. desensitizing.
special instructions
If the symptoms of the disease worsen or there is no effect after 9 days, it is necessary to correct the course of treatment. When using the drug Cocarnit, proper selection of the dose of the hypoglycemic drug and adequate control of the course of diabetes mellitus are necessary. The color of the prepared solution should be pink. Do not use the drug if the color of the solution has changed. The solution must be used immediately after its preparation. Use in pediatrics There are no data on the effectiveness and safety of the drug Cocarnit in children. Impact on the ability to drive vehicles and operate machinery If side effects from the central nervous system (dizziness, confusion) occur, it is recommended to refrain from driving vehicles and other mechanisms.
Dispensing conditions in pharmacies
On prescription
Cocarnit
The drug is a rationally selected complex of metabolic substances and vitamins.
Trifosadenine is an adenosine derivative that stimulates metabolic processes. Has a vasodilating effect, incl. to the coronary and cerebral arteries. Improves metabolism and energy supply to tissues. Has hypotensive and antiarrhythmic effects. Under the influence of ATP, blood pressure decreases, smooth muscles relax, and the conduction of nerve impulses improves.
Cocarboxylase is a coenzyme formed in the body from thiamine (vitamin B1) supplied from outside. It is part of the enzyme carboxylase, which catalyzes the carboxylation and decarboxylation of keto acids. Indirectly promotes the synthesis of nucleic acids, proteins and lipids. Reduces the concentration of lactic and pyruvic acids in the body and promotes the absorption of glucose. Improves trophism of nervous tissue.
Cyanocobalamin (vitamin B12) is converted into methylcobalamin and 5-deoxyadenosylcobalamin in the body. Methylcobalamin is involved in the conversion of homocysteine to methionine and S-adenosylmethionine - key reactions in the metabolism of pyrimidine and purine bases (and, consequently, DNA and RNA). If there is insufficiency of the vitamin in this reaction, it can be replaced by methyltetrahydrofolic acid, and folate-requiring metabolic reactions are disrupted.
5-deoxyadenosylcobalamin serves as a cofactor in the isomerization of L-methylmalonyl-CoA to succinyl-CoA, an important reaction in carbohydrate and lipid metabolism. Vitamin B12 deficiency leads to impaired proliferation of rapidly dividing cells of hematopoietic tissue and epithelium, as well as impaired formation of the myelin sheath of neurons.
Nicotinamide is one of the forms of vitamin PP, participates in redox processes in the cell, improves carbohydrate and nitrogen metabolism, and regulates tissue respiration.
Pharmacokinetics
Trifosadenine
After parenteral administration, it penetrates into organ cells, where it is broken down into adenosine and inorganic phosphate, releasing energy. Subsequently, the breakdown products are included in the resynthesis of ATP.
Cocarboxylase
Rapidly absorbed after intramuscular administration. Penetrates into most tissues of the body. Subject to metabolic decomposition. Metabolic products are excreted primarily by the kidneys.
Cyanocobalamin
In the blood, cyanocobalamin binds to transcobalamins I and II, which transport it to tissues. Deposited mainly in the liver. Communication with plasma proteins 0.9%. It is quickly and completely absorbed after intramuscular and subcutaneous administration. Cmax after intramuscular administration is reached after 1 hour. It is excreted from the liver by bile into the intestines and again absorbed into the blood. T1/2 - 500 days. With normal renal function, 7-10% is excreted by the kidneys, about 50% by the intestines. With reduced renal function - 0-7% by the kidneys, 70-100% - by the intestines. Penetrates through the placental barrier into breast milk.
Nicotinamide
Quickly distributed into all tissues. Penetrates through the placental barrier and into breast milk. Metabolized in the liver to form nicotinamide-N-methylnicotinamide. Excreted by the kidneys.
T1/2 from plasma is about 1.3 hours, steady-state Vd is about 60 l, total clearance is about 0.6 l/min.
Introduction
Diabetes mellitus (DM) is one of the global problems of modern health care, because
causes complications from the nervous and cardiovascular systems, reduces the quality of life, and increases the risk of sudden death [1]. Currently, according to the International Diabetes Federation, 415 million adults (every 11th inhabitant of the globe) suffer from diabetes. According to the Federal Register of Diabetes, in 2016 in Russia, 4.3 million people were registered as suffering from this disease [2, 3]. Diabetic polyneuropathy (DPN), according to various authors, occurs among 50–80% of patients with type 2 diabetes (T2DM) and is associated with severe pain, which leads to a decrease in quality of life. The DIAD study showed that sensory deficits and neuropathic pain are independent risk factors for sudden cardiac death or nonfatal myocardial infarction. The ACCORD study found that a history of neuropathy predicts increased mortality among patients [4]. Distal symmetric sensorimotor polyneuropathy is the most common type of DPN: up to 75% of cases, and, according to some authors, develops already at the stage of prediabetes [5].
The pathogenesis of DPN is currently considered in terms of the interaction between metabolic and vascular factors [6]. It is known that in T2DM, strict glycemic control only slightly reduces the risk of developing neuropathic complications. According to modern studies, factors influencing the development of DPN include the patient’s age, dyslipidemia, arterial hypertension, and smoking [7].
In light of modern data, treatment of DPN should include action on risk factors, pathogenetic and symptomatic therapy, and treatment of diabetes itself. This makes urgent the need for complex treatment of DPN including drugs that affect its pathogenesis [8].
One of the new drugs proposed for the complex pathogenetic and symptomatic treatment of DPN is Cocarnit, containing triphosadenine 10 mg, cyanocobalamin 0.5 mg, nicotinamide 20 mg and cocarboxylase 50 mg.
Purpose of the study: to evaluate the effectiveness of complex therapy, including the drug Cocarnit, for patients with DPN.
Methods
The study included 20 patients with T2DM (mean age 62.35±7.9 years), incl. 14 women (70%) aged 63.2±8.1 and 6 men (30%) aged 60.1±7.9 years. The average duration of diabetes was 11.9±4.7 years (15%<5 years, 60% – 5–15 years, 25%>15 years).
Inclusion criteria for the study: patients of both sexes over the age of 18 years, suffering from DPN, who signed informed consent.
Exclusion criteria: presence of contraindications in accordance with the instructions for use of Cocarnit; polyneuropathy of another origin; taking alpha-lipoic acid, B vitamins and Actovegin for a month before inclusion in the study.
All patients underwent a comprehensive clinical examination, which included general clinical and biochemical blood tests, general urinalysis, electrocardiography, and chest x-ray. Each patient was consulted by a neurologist and an endocrinologist on the 1st (visit 1), 9th (visit 2), 30th (visit 3) and 90th (visit 4) days, each patient underwent electroneuromyography (EMG) on 1 th, 9th and 90th days.
The study of the functional state of peripheral nerves using EMG stimulation was carried out using a 4-channel Viking Quest device (Nicolet Biomedical, USA). Stimulation of the motor nerves (n. peroneus, or peroneal nerve, superficial and deep, n. tibialis, or tibial nerve) was carried out with registration of the amplitude of the motor M-response and the speed of propagation of excitation, distal and residual latency. To assess the condition of the sensory nerves (n. peroneus - superficial and deep, n. suralis, or sural nerve), antidromic stimulation was used to determine the amplitude of the sensory S-response and the speed of propagation of excitation. When analyzing the results obtained, data from 20 healthy patients were used as normal values [9, 10].
The effectiveness of the drug was assessed using the Total Symptom Score (TSS), the Neurological Symptoms Score (NSS) and the Neuropathic Disability Score (NDS).
An examination by a neurologist included a study of knee and Achilles reflexes, tactile, pain, temperature and vibration sensitivity. Vibration sensitivity was studied using a graduated tuning fork with a vibration frequency of 128 Hz. The quality of life of patients was assessed at the first and fourth visits using the Quality of Life Scale (The Short Form 36; SF-36).
Each of the patients received Cocarnit once a day intramuscularly in a dose of 2 ml for 9 days daily in addition to complex therapy. Complex therapy included hypoglycemic and antihypertensive drugs, as well as antiplatelet agents. Antihyperglycemic drugs included insulins of various spectrums of action, tablets (metformin, sulfonylurea, dipeptidyl peptidase-4 inhibitors). Antihypertensive drugs were from various groups (calcium channel blockers, ACE inhibitors, beta-blockers, sartans, centrally acting, diuretics). Antiplatelet therapy in the form of acetylsalicylic acid.
The study did not include patients who received therapy with B vitamins or thioctic acid preparations within 6 months before the start of the research program.
Statistical processing of the obtained results was carried out using Excel Worksheet software from the Microsoft Office 2013 package. The arithmetic mean, standard deviation and reliability criterion (t) were calculated. Differences at the 95% significance level at p<0.05 were accepted as significant.
Research results
In all the patients we examined who suffered from diabetes, distal symmetrical sensorimotor polyneuropathy was identified, a mixed variant (Classification of diabetic neuropathy; Dedov I.I., 2002) of varying severity, which in 25% of cases was combined with radiculopathy, in 10% with mononeuropathy peripheral nerves. All patients experienced neuropathic pain of varying severity, which was confirmed by data from the TSS, NSS and NDS scales. During the therapy, a significant decrease in pain was noted, which is reflected in Table. 1.
The SF-36 questionnaire assessed quality of life, physical, psychological, social and mental health, pain intensity and vital activity. Testing was carried out on the 1st and 4th visits. All patients showed significant improvement on the scales indicated in Table. 2. The most pronounced improvement was observed on the scales of role (physical) functioning (Table 2).
When conducting EMG, manifestations of distal sensory-motor polyneuropathy of varying severity were identified in 100% of patients. The nature of the changes was axonal-demyelinating, with sensory fibers being the most affected. Of the sensory nerves, the most affected is the superficial n. peroneus: the lowest impulse conduction speed and the lowest action potential amplitude were recorded (Tables 3, 4). In 20% of cases, action potentials on the peroneal nerves were not recorded. Compared to the control group, all patients had significantly reduced speed indicators and amplitudes in relation to sensory and motor nerves.
When examining motor nerves, axonal damage was detected in 72% of patients. The lowest amplitudes of the M-response were noted in the study of superficial n. peroneus, which is reflected in table. 5.
Data on conduction velocity along motor nerves are presented in Table. 6.
According to EMG data, the sensory nerves turned out to be the most sensitive to treatment, for which, as a result of therapy, a more pronounced increase in the speed of impulses was noted, although these changes did not reach a degree of statistical significance. In 4 (20%) patients, action potentials on n were not recorded before treatment. peroneus, but after therapy they were not recorded along the peroneal nerves before treatment; after treatment they appeared in 2 of these patients.
Indicators of functional state n. peroneus (sensory fibers) and n. suralis demonstrated the presence of a significant feedback between the amplitude of action potentials of sensory nerves and the duration of diabetes. In patients with diabetes duration >20 years, the lowest potential amplitudes per n were noted. suralis: 1.8±1.2 versus 4.9±3.8 µV in the general group.
A significant inverse relationship was also established between uncontrolled glycemia and damage to the sensory nerves of the lower extremities: 2.1 ± 0.5 (in patients with uncontrolled glycemia) versus 4.9 ± 3.8 μV in the general group.
Discussion
Analysis of our results of examination of patients with DPN indicates a significant decrease in their conduction speed and amplitudes of impulses along the motor and sensory nerves of the lower extremities compared with healthy individuals. Sensory disorders prevailed over motor disorders in the patients we observed, which is consistent with the results obtained by other authors [11].
The nerves were affected by the type of axonomyelinopathy with a predominance of damage by the type of myelinopathy. The lowest impulse conduction velocities and the lowest amplitudes were found in superficial n. peroneus. This indicates the advisability of its inclusion in the EMG examination of patients with T2DM.
As a result of the use of Cocarnit as part of complex therapy, it was possible to improve the conduction along the motor and sensory nerves of the lower extremities, the most pronounced improvements were observed in the more affected sensory nerves. A significant increase in the amplitude of action potentials on the surface n was revealed. peroneus, which indicates the influence of Cocarnit on axonal function. There was also a significant improvement in quality of life indicators and the feeling of neuropathic pain by the patients themselves according to the TSS, NSS and NDS scales. All these data convincingly indicate the positive effect of Cocarnit on the main symptoms of DPN. Regression of neurological deficit during treatment was confirmed by EMG results. The data obtained in this work coincide with the results of previous studies.
Conclusion
Cocarnit is an effective drug for the treatment of patients with diabetic distal sensorimotor polyneuropathy. The use of Cocarnit, containing B vitamins, as well as trifosadenine and nicotinamide, appears to be pathogenetically justified and effective as part of the complex treatment of patients with diabetic neuropathy.
Material and methods
A clinical and neurophysiological examination was carried out on 30 patients with DDSP aged from 27 to 65 years (average - 55.3±10.8 years) and duration of diabetes from 2 to 20 years (average - 8.0±5.8 years). Inclusion criteria
into the study: patients of both sexes over the age of 18 years, suffering from DDSP, who signed informed consent.
Exclusion criteria
: contraindications in accordance with the instructions for use of Cocarnit; polyneuropathy of another origin; taking alpha-lipoic acid, preparations containing B vitamins, and Actovegin for a month before inclusion in the study.
All patients underwent a standard neurological examination with a detailed assessment of pain using the PainDetect pain questionnaire. TSS questionnaires.
.: Total Symptom Score - overall assessment of neuropathy symptoms) and NSS (
English
: Neurological Symptoms Score - assessment of neurological symptoms).
The assessment of quality of life was carried out using the Russian version of the general international questionnaire EuroQol-5D ( English
: European Quality of Life instrument - European Quality of Life Assessment Tool).
In order to study the state of the peripheral neuromotor apparatus, patients underwent stimulation electromyography (EMG) with analysis of conduction along the motor fibers of the peripheral nerves, along the sensory fibers of the peripheral nerves and evoked cutaneous sympathetic potential (ESP). The study was carried out on a 2-channel electromyograph Keypoint Portable (Denmark) using surface lead and stimulating electrodes. The condition of the motor fibers of the median, ulnar, tibial and peroneal nerves was assessed. The amplitude of the motor response (M-response) and the threshold for its registration, the velocity of propagation of excitation (SPV), distal latency (DL) and the state of late responses were analyzed. The following were accepted as the norm: the amplitude of the M-response of the ulnar nerve - no less than 6 mV, the median and tibial nerves - no less than 4 mV, the peroneal nerve - no less than 3 mV; SRV along the motor fibers of the peripheral nerves of the upper extremities - not lower than 50 m/s, of the lower extremities - not lower than 40 m/s; DL for the median and ulnar nerves is 3.5 ms, for the peroneal and tibial nerves – 4 ms [6, 7].
Conduction along sensory fibers was studied on the median, ulnar, sural and superficial peroneal nerves. The SRT and sensory response amplitude (S-response) were determined. The S-response amplitude for the median and ulnar nerves was considered normal to be at least 10 μV, for the sural and superficial peroneal nerves to be at least 5 μV; SRT along the sensory fibers of the peripheral nerves of the upper extremities is not less than 48 m/s, of the lower extremities - not less than 38 m/s [8, 9].
To study the state of autonomic fibers, the amplitude and latency of VCSPs were studied. The amplitude of the VCSP from the palmar surface of the hand was taken to be no lower than 330±102 μV, the latent period was 1.4±0.1 s; from the plantar surface of the foot—amplitude 230.2±81.7 μV, latent period—2.0±0.2 s [10, 11]. Clinical and neurophysiological examination was carried out twice - before drug administration and 2 weeks after the last injection. Cocarnit was injected deeply intramuscularly into the gluteal muscle, 2 ml once daily for 9 days.
Statistical processing of the obtained results was carried out on a PC using Excel Worksheet software from the Microsoft Office 2013 package. The method of variation statistics was used with the calculation of the arithmetic mean ( M
), standard deviation (
SD
), and significance test (
t
).
p
were accepted as significant .
Cocarnit solution for injection, 2 ml in ampoules, 3 pcs. + solvent, 3 pcs.
Nicotinamide
Anticoagulants, acetylsalicylic acid - increased risk of hemorrhage. Caution should be exercised during simultaneous use.
Antihypertensive drugs – enhance the hypotensive effect. Caution should be exercised during simultaneous use.
Antibiotics – possible increase in hyperemia caused by nicotinamide.
Lovastatin, pravastatin - simultaneous use with nicotinamide is not recommended due to the increased risk of adverse reactions. Cases of rhabdomyolysis have been reported with concomitant use of nicotinamide with lovastatin.
Neomycin, barbiturates, anti-tuberculosis drugs, sulfonamides - reducing the toxicity of the latter and preventing neomycin-induced decrease in the concentration of cholesterol and high-density lipoproteins.
Oral contraceptives, isoniazid - a possible increase in the need for nicotinamide (due to a slowdown in the conversion of tryptophan to nicotinic acid).
Probenecid - weakening of the effect of the latter.
Ciprofibrate - simultaneous use with nicotinamide is not recommended.
Fibrinolytic agents, antispasmodics, cardiac glycosides - strengthening of the latter.
Nicotinamide increases the toxic effect of alcohol on the liver.
Cocarboxylase
Cardiac glycosides – enhancing the cardiotonic effect of cardiac glycosides.
Cyanocobalamin
Drugs that increase blood clotting - simultaneous use with nicotinamide is not recommended.
Oral contraceptives - decrease in the concentration of cyanocobalamin in the blood plasma.
Thiamine – increased risk of allergic reactions caused by thiamine.
Chloramphenicol – decreased hematopoietic response to the drug.
Antimetabolites and most antibiotics alter the results of microbiological studies of cyanocobalamin.
Disodium adenosine triphosphate trihydrate
β-adrenergic receptor blockers, nitrates – enhance the antianginal effect.
Dipyridamole – enhances the action of the latter, in particular its vasodilating effect.
Potassium-sparing diuretics, potassium supplements, ACE inhibitors – increased risk of hyperkalemia.
Carbamazepine – enhances the effect of adenosine (including before the development of blockade).
Xanthinol nicotinate – weakening the effect of adenosine.
Purine derivatives (caffeine and theophylline) - some antagonism with adenosine is revealed.
Magnesium supplements – increased risk of hypermagnesemia.
Cardiac glycosides – increased risk of adverse reactions from the cardiovascular system. The drug should not be administered in large doses simultaneously with cardiac glycosides.
Lidocaine hydrochloride
Amitriptyline, bupivacaine, disopyramide, imipramine, nortriptyline, pethidine, quinidine, chlorpromazine - decrease in the concentration of lidocaine in the blood plasma.
Antiarrhythmic drugs (including amiodarone, verapamil, quinidine, disopyramide, ajmaline) - increased cardiodepressive effect (due to prolongation of the QT interval) and in very rare cases, the development of atrioventricular block or ventricular fibrillation is possible. Concomitant use with amiodarone may lead to the development of seizures.
Anticoagulants (including ardeparin, dalteparin, danaparoid, enoxaparin, heparin, warfarin) - increase the risk of bleeding.
Acetazolamide, thiazide and loop diuretics – weakening of the effect of lidocaine (due to hypokalemia).
Barbiturates (phenobarbital), anticonvulsants - increase metabolism and decrease the concentration of lidocaine in the blood plasma, as well as enhance the cardiodepressive effect.
β-adrenergic receptor blockers - slowing down metabolism and increasing the effects (including toxic) of lidocaine, in particular, increasing the risk of bradycardia and arterial hypotension. If these drugs are used simultaneously, the dose of lidocaine should be reduced.
Vasoconstrictors (epinephrine, methoxamine, phenylephrine) - may slow absorption and prolong the effect of lidocaine.
Glucagon, isadrin – increase the clearance of lidocaine.
Guanadrel, guanethidine, mecamylamine, trimetaphan - with spinal and epidural anesthesia, the risk of severe arterial hypotension and bradycardia increases.
Anesthesia (hexobarbital, sodium thiopental intravenously), ethanol - increased inhibitory effect on breathing.
Drugs that predetermine the blockade of neuromuscular transmission - enhancing the effect of such drugs (as a result of reducing the conductivity of nerve impulses).
Monoamine oxidase inhibitors (furazolidone, procarbazine, selegiline) – increase the risk of arterial hypotension. During treatment with MAO inhibitors, lidocaine should not be used parenterally.
Curare-like drugs – deepening muscle relaxation (up to paralysis of the respiratory muscles).
Mexiletine, norepinephrine - increased toxicity of lidocaine (as a result of decreased clearance and hepatic blood flow).
Midazolam - increases the concentration of lidocaine in the blood plasma.
Narcotic analgesics (morphine) - enhance the analgesic effect of such drugs, but respiratory depression also increases.
Novocaine, novocainamide, procainamide - central nervous system (CNS) stimulation, delirium, hallucinations.
Polymyxin B - with simultaneous use, respiratory function should be monitored.
Prenylamine – increases the risk of ventricular arrhythmias.
Propafenone – increased duration and increased severity of CNS side effects.
Rifampicin - a decrease in the concentration of the latter in the blood plasma.
Sedatives and hypnotics – enhance the inhibitory effect on the central nervous system.
Cardiac glycosides – weakening of the cardiotonic effect of cardiac glycosides. Against the background of intoxication with digitalis glycosides, lidocaine can aggravate the severity of AV block.
Cimetidine - decreased metabolism (decreased hepatic clearance due to inhibition of microsomal oxidation) and an increase in the concentration of lidocaine in the blood plasma, as well as an increase in its toxic effects.
Possibility of using the drug Cocarnit in the treatment of diabetic polyneuropathy
Diabetic polyneuropathy is a complication of diabetes mellitus that develops, along with other complications (angiopathy), as a result of chronic hyperglycemia. Achieving normoglycemia is the main condition for the prevention and treatment of diabetic complications. Unfortunately, it is possible to maintain normal blood glucose levels in a small number of patients, which dictates the need to search for drugs that prevent the toxic effect of glucose on tissues. One of these drugs is Cocarnit, which has proven its effectiveness in a number of studies.
Table 1. Dynamics of neuropathic symptoms according to TSS during therapy with Cocarnit, %
Table 2. Dynamics of neuropathic symptoms according to NIS-LL during therapy with Cocarnit, %
Table 3. Dynamics of asthenia symptoms according to MFI-20 during therapy with Cocarnit, score
Drawing. Number of patients who showed improvement according to GCIC at the end of follow-up
Diabetic polyneuropathy (DPN) is a complication of diabetes mellitus (DM), which, along with other complications (angiopathy), is a consequence of chronic hyperglycemia. The disease causes the development of diabetic foot syndrome (ulcer, gangrene, pseudarthrosis), painless forms of myocardial infarction, damage to the gastrointestinal tract and urogenital disorders.
Diabetic peripheral polyneuropathy (DPPN) is characterized by a complex of clinical manifestations, has clear diagnostic criteria and serves as a marker of visceral, in particular life-threatening cardiac, diabetic neuropathy [1, 2].
Currently, there are no clear data on the prevalence of DPN. It is believed that on average more than 50% of patients with diabetes suffer from this disease. Among the risk factors for development are the duration of diabetes, the duration of periods of severe hyperglycemia, the level of glycated hemoglobin, the presence of cardiovascular pathology, arterial hypertension, alcohol consumption, and smoking. Thus, the prevalence of DPPN with diabetes duration up to five years and more than 30 years increases from 14 to 44%, respectively. The risk of developing DPN increases by 10–15% with a 1 mmol/L increase in fasting glucose or 1% increase in glycated hemoglobin.
At the moment, there are two complementary hypotheses for the development of DPN: metabolic and vascular. However, it must be remembered that a key role in pathogenesis belongs to chronic hyperglycemia, which triggers a cascade of biochemical reactions leading to degeneration and demyelination of nerve fibers. Hypoglycemia also contributes to the development of DPN. Thus, frequent episodes of severe hypoglycemia are associated with demyelination of the nerve fiber and pathology of the anterior horn of the gray matter of the spinal cord [3].
Some scientists believe that in the early stages of the development of the disease, metabolic factors predominate; they also prevail in diffuse nerve damage; in the later stages and in focal neuropathies, the role of vascular factors increases [4].
The metabolic theory of pathogenesis is based on the activation of the polyol pathway of glucose metabolism, which leads to the accumulation of sorbitol. As a consequence, swelling of the tissues of both the nervous and vascular walls, disruption of endoneurial blood flow and hypoxia of the nerves. It is important to note that normally only 1–2% of glucose is converted into sorbitol; under conditions of hyperglycemia, the rate of this process increases seven to ten times.
Activation of the polyol pathway also affects the formation of nitric oxide (NO) inside cells. It is known that when the concentration of this highly active radical decreases, intraneural blood flow decreases, as a result, the speed of excitation along the nerve fiber slows down. This mechanism determines the initial changes in the peripheral nervous system in response to hyperglycemia [4–6].
Another pathobiochemical pathway of damage is an increase in the formation of advanced glycation end products. This leads to disruption of the structure of the basement membrane of capillaries, intracellular proteins, including mitochondrial, and glucose oxidation processes with the development of oxidative stress [7, 8].
In recent years, oxidative stress has been given a special place in the pathogenesis of DPN. Increased production of free radicals and decreased antioxidant defense are important pathobiochemical characteristics of diabetes. Oxidative stress underlies the development of both microvascular complications and the progression of atherosclerosis [7, 9].
Clinically, DPPN is manifested by a decrease in the sensitivity of all modalities in the distal parts of the legs, Achilles and knee reflexes, and weakness of the muscles of the lower leg and foot [3, 10].
In addition, very often chronic diseases, including diabetes, are accompanied by asthenovegetative syndrome (fatigue sindrom). It is characterized by increased fatigue, weakness, irritability, inability to concentrate, decreased satisfaction with life, decreased performance, and causes a deterioration in the quality of life of patients and decreased compliance with treatment [11].
The peculiarity of the symptoms of DPPN makes diagnosis difficult, as a result, time is lost and the processes become irreversible. According to a survey of surgeons involved in the surgical treatment of patients with diabetic foot syndrome, 43–85% of those who underwent limb amputation could have avoided this if preventive therapy had been started in a timely manner [4].
Today, pathogenetic therapy of the disease involves taking drugs that affect oxidative stress and B vitamins [3, 4, 10].
Purpose
The present study was to evaluate the effect of the drug Cocarnit (lyophilisate for preparing a solution for intramuscular administration) when used once a day intramuscularly in a dose of 2 ml for nine days on the dynamics of neuropathic symptoms in patients with diabetic polyneuropathy.
Research objectives:
- studying the effect of the drug Cocarnit on neuropathic symptoms;
- assessment of the effect of the drug Cocarnit on the overall clinical impression of changes in symptoms of neuropathy and the frequency of adverse reactions.
Material and methods
The study included 34 patients (14 men and 19 women) with diabetes mellitus: type 1 diabetes in 4, type 2 diabetes in 29. The average age of the participants was 53.6 ± 2.4 [24.0–74.0] years, duration of diabetes – 9.8 ± 0.1 [1.0–31.0] years.
Each participant signed an informed consent for inclusion in the clinical study and processing of examination results.
All patients received basic glucose-lowering therapy: insulin, oral hypoglycemic drugs, antihypertensive drugs, drugs for the treatment of concomitant diseases. Cocarnit was added to the treatment regimen.
Taking medications containing B vitamins and alpha-lipoic acid was not allowed.
The drug Cocarnit is a rationally selected complex of metabolic substances and vitamins: triphosadenine disodium trihydrate, cocarboxylase, cyanocobalamin, nicotinamide.
Trifosadenine disodium trihydrate is an adenosine derivative that stimulates metabolic processes. The substance has a vasodilating effect, including on the coronary arteries and cerebral arteries. Improves metabolism and energy supply to tissues. Has hypotensive and antiarrhythmic effects. Under the influence of adenosine triphosphate, blood pressure decreases, smooth muscles relax, and the conduction of nerve impulses improves.
Cocarboxylase is a coenzyme formed in the body from thiamine (vitamin B1) supplied from outside. Part of the enzyme carboxylase, which catalyzes the carboxylation and decarboxylation of alpha-keto acids. Indirectly promotes the synthesis of nucleic acids, proteins and lipids. Reduces the concentration of lactic and pyruvic acids in the body, promotes the absorption of glucose. Improves trophism of nervous tissue.
Cyanocobalamin (vitamin B12) is converted into methylcobalamin and 5-deoxyadenosylcobalamin in the body. Methylcobalamin is involved in the conversion of homocysteine to methionine and 8-adenosylmethionine, a key reaction in the metabolism of pyrimidine and purine bases (hence DNA and RNA). In case of vitamin deficiency, methyltetrahydrofolic acid can replace it, and folic-requiring metabolic reactions are disrupted. 5-deoxyadenosylcobalamin serves as a cofactor in the isomerization of L-methylmalonyl-CoA to succinyl-CoA, an important reaction in carbohydrate and lipid metabolism.
Vitamin B12 deficiency leads to impaired proliferation of rapidly dividing cells of hematopoietic tissue and epithelium, as well as the formation of the myelin sheath of neurons.
Nicotinamide is one of the forms of vitamin PP, participates in redox processes in the cell, improves carbohydrate and nitrogen metabolism, and regulates tissue respiration.
The regimen for using the drug Cocarnit involved intramuscular administration of 2 ml for nine days, then on the 12th, 15th and 18th days. The course of therapy is 12 injections.
The main criteria for assessing the effectiveness of the study drug:
- dynamics of neuropathic symptoms according to TSS (Total Symptom Score - general assessment of neuropathy symptoms);
- neuropathic symptoms according to NIS-LL (Neuropathy Impairment Score - Lower Limb - scale of neuropathic disorders of the lower extremities);
- MFI-20 indicators (Multidimensional Fatigue Inventory - a subjective scale for assessing asthenia).
Secondary indicators:
- dynamics of the general clinical impression of changes in neuropathy symptoms according to the GCIC (Global Clinical Impression Change) questionnaire;
- frequency of side effects of therapy.
The duration of observation was 12 weeks.
Other treatments prescribed during the study were noted in the individual patient registration record.
The study design involved three visits. The first is inclusion in the study (first day), the second is three days after the end of the course of injections (21st day), the third is 42 days from the start of therapy.
At all visits, patients were assessed using TSS, NIS-LL, GCIC and MFI-20. In addition, during the first and second visits, standard general and biochemical blood tests were carried out; on the third, a general conclusion was made about the effectiveness of treatment: marked improvement, moderate improvement, minimal improvement, no dynamics, slight deterioration, moderate deterioration, significant deterioration, as well as presence of adverse reactions.
Based on the results obtained, the arithmetic mean (M), standard error (m), and relative value (p, %) were calculated. To confirm the significance of differences between samples, Student's t-test was used.
The probability of error was indicated as p and was considered acceptable when
Statistical data processing was carried out using the Statistica 6.0 program.
results
All patients included in the study had clinical manifestations of DPPN. Thus, 60.6% of patients complained of moderately severe pain, burning, paresthesia, numbness, 36.3% – their severe manifestations (according to TSS) (Table 1). After 21 days, during therapy with Cocarnit, 54.1% of patients showed a decrease in the symptoms of DPPN to normal, and in 45.4% - to a moderately severe degree. To date, severe symptoms of the disease have not been observed in any patient (Table 1). Positive dynamics were also observed during the third visit.
The average TSS scores were: first visit – 6.32 ± 0.8 points (p
Assessment of neuropathic deficit according to NIS-LL (muscle strength, reflexes, tactile, temperature, vibration, proprioceptive sensitivity) showed that initially the proportion of patients with severe neuropathy was 21.2%, moderate - 69.8% (Table 2). As a result of the therapy, the share of the former decreased to 9.1%, and the latter to 48.4%. At the same time, the number of patients with no neuropathic deficit by the end of the study increased from 9.1 to 30.3% (first and third visits, respectively) (Table 2). It should be noted that the most pronounced treatment effect was observed after 21 days (second visit). After 42 days, a slowdown in positive dynamics was recorded, but the number of patients with moderate neuropathy remained high.
Average NIS-LL scores: first visit – 10.1 ± 0.8 points, second – 6.8 ± 0.7 points (p
The dynamics of asthenia manifestations were determined using MFI-20. This questionnaire includes 20 questions to assess general, physical, mental asthenia, and decreased motivation in the study group. Table 3 shows the changes in the physical and mental state of patients during treatment with Cocarnit. Reducing the symptoms of the disease made it possible to expand the physical capabilities of patients, improve their general condition and emotional state.
According to GCIC, therapy with Cocarnit helps improve neuropathic symptoms. The general conclusion about the effectiveness of treatment is presented in the figure.
Regarding side effects and complications in patients during the course of therapy, the following should be noted. One patient out of 35 dropped out of the study because after the first injection she developed an allergic reaction in the form of a rash. The remaining participants did not experience any side effects.
Conclusion
The study showed that patients with diabetes should be regularly examined for signs of DPPN. Long-term unsatisfactory compensation of diabetes leads to the development of complications.
To prevent more severe damage to the nervous system, the development of diabetic foot syndrome and cardiopathy, it is necessary to carry out preventive as well as therapeutic measures (at the stage of clinical manifestations of the disease).
B vitamins belong to neurotropic drugs and are recognized as pathogenetic therapy for DPN [4, 6].
The results of the study also indicate that treatment with Cocarnit is associated with a pronounced and moderate improvement in neuropathic symptoms and the general condition of patients.