Guillain-Barre syndrome - symptoms and treatment
Patients with GBS are at risk for life-threatening respiratory complications and autonomic dysfunction.
Indications for transfer to the intensive care unit include:
- rapid progression of motor weakness with damage to the respiratory muscles;
- ventilation respiratory failure;
- pneumonia;
- bulbar disorders;
- severe autonomic failure.
Complications of treatment requiring intensive care include fluid overload, anaphylaxis to intravenous immunoglobulin, or hemodynamic compromise during plasmapheresis.
15%-25% of children with GBS develop decompensated respiratory failure, which requires mechanical ventilation.[2] Respiratory disorders are more common in children with rapid disease progression, upper limb weakness, autonomic dysfunction, and cranial nerve damage. Tracheal intubation may be required in patients to protect the respiratory tract and provide mechanical ventilation. In GBS, rapid progression, bilateral facial palsy and autonomic dysfunction predetermine an increased likelihood of intubation. Planning for early intubation is necessary to minimize the risk of complications and the need for emergency intubation.
Autonomic dysfunction increases the risk of endotracheal intubation. On the other hand, dysautonomia may increase the risk of hemodynamic reactions to drugs used to induce anesthesia during intubation.
Signs indicating the need for mechanical ventilation:[4]
- ventilation respiratory failure;
- increased oxygen demand to maintain SpO2 above 92%;
- signs of alveolar hypoventilation (PCO2 above 50 mmHg);
- rapid decrease in vital capacity by 50% compared to the initial level;
- inability to cough
Autonomic dysfunction is the main factor of mortality in GBS. Fatal cardiovascular collapse due to autonomic dysfunction occurs in 2% to 10% of critically ill patients.[3] Heart rate, blood pressure, and electrocardiogram monitoring should continue as long as patients require respiratory support. Transcutaneous pacing may be required for severe bradycardia. Hypotension is corrected by replenishing the circulating blood volume (CBV), and if the patient is refractory to replenishing the circulating blood volume, α-agonists such as norepinephrine, mesaton, and adrenaline are used.
In unstable hemodynamics, continuous recording of arterial and central venous pressure should be carried out to monitor the volume of infusion therapy.
Hypertension may occur, but this complication does not require specific treatment unless it is complicated by pulmonary edema, encephalopathy, or subarachnoid hemorrhage.
Causes
Guillain-Barré syndrome is often preceded by some kind of infection, which can be bacterial or viral. The development of Guillain-Barre syndrome can also be triggered by vaccination or surgery.
In the context of Zika virus infection, an unexpected increase in the number of cases of Guillain-Barré syndrome has been noted in affected countries. The most likely explanation for the available evidence regarding outbreaks of Zika virus infection and Guillain-Barré syndrome is that Zika virus infection is a contributing cause of Guillain-Barré syndrome.
Symptoms of the disease
Guillain-Barré syndrome has three forms:
- acute - symptoms appear already on the 1st-3rd day;
- subacute - a person may not be aware of the illness for up to 3 weeks;
- chronic - flows sluggishly, symptoms appear weakly. Difficult to treat.
In the first days, the patient experiences symptoms characteristic of ARVI:
- aches, numbness in the limbs;
- temperature increase;
- weakness, lethargy, headache.
Nausea, vomiting, and diarrhea may be present. Because of this, a person may not immediately guess about serious disorders in the body. And he uses antiviral, anti-inflammatory drugs, which are useless for nerve damage.
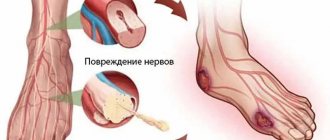
Further, specific signs of the syndrome appear. First of all, this is weakness in the limbs. Due to damage to nerve cells, muscles lose sensitivity. As a rule, discomfort first appears in the legs, then spreads to the arms. A characteristic symptom is symmetrical damage to the extremities - two arms or legs begin to bother at once.
The second sign is a protruding belly. It increases because the patient is forced to breathe abdominally due to a weakening of the diaphragm.
The functioning of the muscles of the pharynx and jaws is disrupted: it becomes difficult for a person to chew and swallow food. In the acute form of the disease, limb paralysis develops.
Diagnostics
Diagnosis is based on symptoms and neurological examination findings, including decreased or loss of deep tendon reflexes. A spinal tap may be performed to obtain supporting information, but this should not lead to a delay in treatment.
Diagnosing Guillain-Barré syndrome does not require other tests, such as blood tests, to determine the cause of the syndrome, and these tests should not lead to delays in treatment.
Publications in the media
Guillain–Barré syndrome is a post-infectious demyelinating polyneuropathy, the most common demyelinating lesion. It is characterized by peripheral paralysis of the muscles of the limbs and protein-cell dissociation in the cerebrospinal fluid while maintaining superficial sensitivity. May have an ascending nature involving the muscles of the face, pharynx, and larynx (ascending Landry's palsy). Frequency: 0.6–1.9 cases per 100,000 population. The predominant gender is male.
Etiology. There is an opinion about the autoimmune nature of the disease. Develops after or during the following conditions: • Infectious diseases •• Upper respiratory tract infections •• Infectious mononucleosis •• CMV infection •• Herpes infection •• Influenza A •• Mycoplasma infection •• Mumps •• HIV infection • Lymphoma (especially Hodgken) • Vaccination, serum sickness • Surgery.
Pathogenesis. Selective demyelination of the spinal cord roots, apparently of an autoimmune nature. Against the background of immune disorders, edema, inflammatory cell infiltration and diffuse primary segmental demyelination occur primarily in the anterior roots and proximal parts of the spinal nerves, plexuses, limb nerves and autonomic ganglia.
Pathomorphology • Segmental demyelination of peripheral nerves, in severe cases - axonal degeneration • Lymphocytic and monocyte infiltration of the myelin sheath and perivascular region.
Clinical presentation • Typically, approximately 2 weeks after viral infection or immunization, muscle weakness of the distal lower extremities develops suddenly. In 65% of cases, the disease manifests itself within 3 weeks after the infectious disease • Subsequently, muscle weakness spreads upward to the muscles of the arms, torso, neck, and cranial muscles—symmetrical flaccid tetraparesis is formed. In some cases, only the lower extremities or cranial nerves are affected • Sensory disorders are minimal, pain, parasthesia, hypoalgesia or hyperalgesia in the distal extremities are possible • Paresis of facial muscles and bulbar disorders (bilateral paresis of the oropharyngeal muscles) often occur • Paralysis of the respiratory muscles (5–10 % of cases) • Decrease and then loss of deep tendon reflexes • Autonomic disorders •• Inappropriate secretion of ADH (urinary retention) •• Arrhythmias •• Fluctuations in blood pressure.
Special studies • Electromyography - a significant decrease in the amplitude of the muscle response when stimulating the distal peripheral nerve. Conduction of nerve impulses is slowed • Lumbar puncture. An increase in protein content, sometimes significant (>10 g/l), begins a week after the manifestation of the disease, with a maximum of 4–6 weeks.
Differential diagnosis • Intoxication leading to disruption of neuromuscular transmission •• Poisoning with heavy metals (lead, arsenic) •• Poisoning with industrial substances (acrylamide, carbon disulfide, trichlorethylene, rapeseed oil, organophosphorus compounds) •• Intoxication when taking drugs: gold preparations ... nerves in oncological diseases • Infectious diseases •• Acute poliomyelitis •• Diphtheria (complicated by paralysis) •• Botulism.
Treatment • According to indications - mechanical ventilation (in 10–23% of cases), tracheostomy • Intravenous administration of immunoglobulin (0.4 g/kg/day for 5 days) • In severe cases - plasmapheresis • Sufficient fluid intake to maintain diuresis at level 1 –1.5 l/day under the control of serum electrolyte concentrations • Physiotherapy to prevent contractures • Prednisolone up to 80–120 mg/day orally is prescribed only for chronic inflammatory dimyelinating polyradiculoneuropathy • Syndromic therapy • Heparin 5,000 units subcutaneously 2 times a day - for the entire period of bed rest.
Complications • During the disease: paralysis, respiratory failure and aspiration, arterial hypertension or hypotension, arrhythmias, urinary retention, depression • Consequences of the development of the chronic stage: chronic inflammatory demyelinating polyradiculoneuropathy.
Course and prognosis • Characterized by an acute onset and progressive course. Typically, the progression of the process continues for 2–4 weeks, then gradually, over about a year, functional restoration occurs • In 7–22% of patients, residual neurological deficits are detected (weakness, decreased reflexes) • In 10%, a relapse occurs within one year after recovery or severe complications develop • Mortality - about 3% • The prognosis for unclear etiology is favorable in approximately 50% of cases.
Synonyms • Guillain–Barré–Strol syndrome • Acute primary idiopathic polyradiculoneuritis • Infectious idiopathic polyneuropathy • Radiculanglionitis • Landry–Guillain–Barré syndrome • Acute demyelinating polyradiculoneuropathy Guillain–Barré
ICD-10 • G61.0 Guillain–Barré syndrome
Application. Miller Fisher syndrome is an atypical variant of Guillain–Barré syndrome, in which flaccid distal tetraparesis with areflexia is combined with bilateral external or total ophthalmoplegia and cerebellar ataxia.
WHO activities
WHO is supporting countries in the management of Guillain-Barré syndrome in the context of Zika virus infection by:
- improving surveillance of Guillain-Barré syndrome in countries affected by Zika virus;
- providing guidelines for the assessment and management of Guillain-Barré syndrome;
- supporting countries to implement guidelines and strengthen health systems to better manage cases of Guillain-Barré syndrome;
- defining a research agenda for Guillain-Barré syndrome.
Treatment and care
The following are recommendations for the treatment and care of patients with Guillain-Barré syndrome:
- Guillain-Barre syndrome is potentially life-threatening. Patients with Guillain-Barré syndrome are usually hospitalized and closely monitored.
- Symptomatic treatment includes monitoring breathing, heart rate and blood pressure. If breathing becomes compromised, the patient is usually placed on a ventilator and monitored for complications, which may include abnormal heartbeats, infections, blood clots, and high or low blood pressure.
- There is no cure for Guillain-Barré syndrome, but treatment can reduce the symptoms of Guillain-Barré syndrome and shorten their duration.
- Given the autoimmune nature of the disease, immunotherapy such as plasma replacement to remove antibodies from the blood or intravenous immunoglobulin is usually given during its acute stage. This treatment is most effective when given 7 to 14 days after symptoms begin.
- If muscle weakness persists after the acute stage of the disease, patients may require rehabilitation services to strengthen muscles and restore motor function.