Hopanthenic acid 500 mg, 50 tablets (Ozone)
Registration Certificate Holder
ATOLL (Russia)
Dosage form
Medicine - Hopantenic acid
Description
Pills
white or almost white, round, flat-cylindrical, with a chamfer and a notch.
1 tab.
calcium hopantenate 500 mg
Excipients
: magnesium hydroxycarbonate - 93.6 mg, povidone K25 - 20.2 mg, calcium stearate - 6.2 mg.
10 pieces. — cellular contour packages (1) — cardboard packs. 10 pieces. — contour cell packaging (2) — cardboard packs. 10 pieces. — cellular contour packages (3) — cardboard packs. 10 pieces. — contour cell packaging (4) — cardboard packs. 10 pieces. — contour cell packaging (5) — cardboard packs. 10 pieces. — contour cell packaging (10) — cardboard packs. 20 pcs. — cellular contour packages (1) — cardboard packs. 20 pcs. — contour cell packaging (2) — cardboard packs. 20 pcs. — cellular contour packages (3) — cardboard packs. 20 pcs. — contour cell packaging (4) — cardboard packs. 20 pcs. — contour cell packaging (5) — cardboard packs. 20 pcs. — contour cell packaging (10) — cardboard packs. 25 pcs. — cellular contour packages (1) — cardboard packs. 25 pcs. — contour cell packaging (2) — cardboard packs. 25 pcs. — cellular contour packages (3) — cardboard packs. 25 pcs. — contour cell packaging (4) — cardboard packs. 25 pcs. — contour cell packaging (5) — cardboard packs. 25 pcs. — contour cell packaging (10) — cardboard packs. 30 pcs. — cellular contour packages (1) — cardboard packs. 30 pcs. — contour cell packaging (2) — cardboard packs. 30 pcs. — cellular contour packages (3) — cardboard packs. 30 pcs. — contour cell packaging (4) — cardboard packs. 30 pcs. — contour cell packaging (5) — cardboard packs. 30 pcs. — contour cell packaging (10) — cardboard packs. 10 pieces. - cans (1) - cardboard packs. 20 pcs. - cans (1) - cardboard packs. 25 pcs. - cans (1) - cardboard packs. 30 pcs. - cans (1) - cardboard packs. 50 pcs. - cans (1) - cardboard packs. 100 pieces. - cans (1) - cardboard packs.
Indications
Cerebrovascular insufficiency caused by atherosclerotic changes in cerebral vessels, senile dementia (initial forms), residual organic brain damage in mature and elderly people, cerebral organic insufficiency in patients with schizophrenia, extrapyramidal hyperkinesis in patients with hereditary diseases of the nervous system (including Huntington's chorea, hepatocerebral dystrophy, Parkinson's disease), residual effects of previous neuroinfections, post-vaccination encephalitis, traumatic brain injury (as part of complex therapy); extrapyramidal neuroleptic syndrome (hyperkinetic and akinetic), as a corrector of the side effects of antipsychotics (neuroleptics) and for preventive purposes at the same time as “cover therapy”; epilepsy (with slow mental processes in combination with anticonvulsants). Psycho-emotional overload, decreased mental and physical performance (increased concentration and memory). Urinary disorders: enuresis, daytime urinary incontinence, pollakiuria, urgency (adults and children over 2 years old).
Children: perinatal encephalopathy, mental retardation (delayed mental, speech, motor development or a combination thereof), cerebral palsy, stuttering (mainly clonic form), epilepsy (as part of combination therapy with anticonvulsants, especially with polymorphic seizures and petit mal seizures) .
Contraindications for use
Acute severe kidney disease, first trimester of pregnancy.
pharmachologic effect
A nootropic agent that has neurometabolic, neuroprotective and neurotrophic properties. Increases the brain's resistance to hypoxia and the effects of toxic substances, stimulates anabolic processes in neurons, combines a moderate sedative effect with a mild stimulating effect, has an anticonvulsant effect, reduces motor excitability while regulating behavior. Increases mental and physical performance. Helps normalize GABA content during chronic alcohol intoxication and subsequent ethanol withdrawal. Shows analgesic effect.
Drug interactions
Prolongs the effect of barbiturates, enhances the effects of anticonvulsants, nootropics and central nervous system stimulants, and the effect of local anesthetics (procaine).
Prevents side effects of phenobarbital, carbamazepine, antipsychotics (neuroleptics).
The effect of hopantenic acid is enhanced in combination with glycine and xydiphone.
Dosage regimen
Taken orally. Single dose for adults - 0.5-1 g, for children - 0.25-0.5 g; daily dose for adults - 1.5-3 g, for children - 0.75-3 g. The course of treatment is 1-4 months, in some cases - up to 6 months. After 3-6 months, a second course of treatment is possible.
For children with mental impairment and mental retardation - 0.5 g 4-6 times a day daily for 3 months; for delayed speech development - 0.5 g 3-4 times a day for 2-3 months.
As a corrector for neuroleptic syndrome, adults - 0.5-1 g 3 times/day, children - 0.25-0.5 g 3-4 times/day. The course of treatment is 1-3 months.
For epilepsy, children - 0.25-0.5 g 3-4 times a day, adults - 0.5-1 g 3-4 times a day, daily, for a long time (up to 6 months).
For tics in adults - 1.5-3 g/day, daily, for 1-5 months; children - 0.25-0.5 g 3-6 times a day daily for 1-4 months.
For urinary problems in adults - 0.5-1 g 2-3 times a day, daily dose - 2-3 g; for children, a single dose is 0.25-0.5 g, daily - 25-50 mg/kg. The course of treatment is 0.5-3 months.
Side effect
Allergic reactions:
rhinitis, conjunctivitis, skin rashes.
special instructions
Long-term therapy with hopantenic acid in combination with other nootropic and central nervous system stimulants is not recommended.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated.
Use is contraindicated in the first trimester of pregnancy.
Use for renal impairment
Restrictions for impaired renal function - Contraindicated.
The drug is contraindicated in acute severe kidney disease.
Use in children
Restrictions for children - No restrictions.
Application is possible according to the dosage regimen.
Prospects for the use of the drug Gopantomide in pediatric neurology
Hopantenic acid preparations are widely used in clinical practice for a wide range of diseases of the nervous system. Like most other drugs acting on the nervous system, hopantenic acid and its salts were initially used in adults and later, with numerous evidence of safety and effectiveness in adults, they became widely used in children.
Efficacy of hopantenic acid as a concomitant therapy in the treatment of epilepsy.
The effectiveness and safety of the use of hopantenic acid as a concomitant drug for epilepsy has been shown in studies involving adults and children [2–4, 10–12, 23]. Gopantomide enhances the effects of AEDs, prolongs the effect of barbiturates and improves the tolerability of some AEDs. The use of Gopantomide can largely prevent the side effects of phenobarbital, carbamazepine, and antipsychotics, which helps improve the tolerability of antiepileptic therapy in patients with epilepsy [6, 7].
In accordance with the instructions for medical use, Gopantomide can be used in complex therapy of epilepsy, in combination with AEDs, in the following dose: in adults - from 500 to 1000 mg 3 times a day; in children - from 250 to 500 mg 3-4 times a day. The course of treatment can last up to 6 months. It is possible to conduct repeated courses with a break between courses of 3–6 months [7].
V. I. Guzeva et al. (2007) used the drug hopantenic acid in the form of syrup in 20 children aged 3 years to 4 years 11 months suffering from epilepsy in combination with cognitive impairment and ADHD.
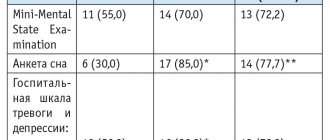
All patients underwent a comprehensive examination, including the use of a structured questionnaire, tests for attention (“extra object”), mechanical memory (memorizing 3–4 words), dynamic memory (repetition of 2 groups of words), Luscher color test (from 4 years old) , as well as EEG. After a course of treatment with hopantenic acid in the form of syrup, an improvement in indicators of attention, mechanical and dynamic memory, a decrease in the degree of anxiety and an increase in the level of compensation according to the Luscher test were revealed in children with epilepsy in combination with ADHD. In most patients, the background EEG rhythm improved. Analysis of the results allowed us to conclude that the drug hopantenic acid in the form of syrup is effective in complex therapy for children with epilepsy combined with cognitive impairment and ADHD. The authors noted the safety and good tolerability of therapy with the drug hopantenic acid in children with epilepsy [10].
S. V. Balkanskaya et al. (2007, 2008) demonstrated the feasibility of using hopantenic acid to improve the psychosomatic health and quality of life of children with rolandic epilepsy. The study involved 21 children (12 boys and 9 girls) aged 6 to 8 years with rolandic epilepsy. The comparison group consisted of 28 practically healthy children of primary school age. The study was conducted on the basis of the psychoneurological department of the Research Institute of Pediatrics of the Scientific Center for Children's Health of the Russian Academy of Medical Sciences. The level and structure of cognitive disorders were assessed using the “Psychomat”, “Binatest”, “Mnemotest” computer test systems; All children underwent EEG. A neuropsychological examination using computer systems revealed the presence of a moderately severe partial deficit in cognitive functions in the majority of those examined. Only 29% of children had no deviations from the age norm (comparison group). The most affected functions in patients with Rolandic epilepsy were those characterizing the quality of analytical and synthetic processes: attention distribution, short-term visual memory, imaginative thinking, and the pace of psychomotor activity. Hopantenic acid was used at a dose of 30 mg/kg/day (daytime, in 2 doses), the course duration was 2 months. Repeated examinations were carried out at intervals of 3–4 months. The positive effect of the drug hopantenic acid on the studied indicators of cognitive functions has been demonstrated. Various degrees of clinical effect of nootropic correction in changing leading complaints have been established: reduction in headache intensity - in 24% of children, reduction in fatigue - in 43%, improvement in sleep - in 5%, improvement in behavior - in 24%, improvement in attention - in 28.5 % of children. A study of the dynamics of psychophysiological functions demonstrated an increase in the quality of memorization processes when studying the volume of short-term visual memory (in the mode without limiting the exposure of the light image) - the volume increased by 21.7%; an improvement in the level of attention was also noted (by 29%). Analysis of the distribution and switching of attention, characterizing the quality of analytical and synthetic processes, in terms of restructuring the decision-making strategy under conditions of choice, revealed an improvement in indicators by 26% after a course of taking the drug. Obviously, the greatest difficulties for children with Rolandic epilepsy were the transformation of the visual image in space; Dynamics showed an improvement in this indicator to the age norm in the majority of children after completing a course of taking hopantenic acid [2–4].
It is important to note that the drug hopantenic acid does not enhance epileptic activity on the EEG, which predetermines the possibility of its use both in concomitant therapy for epilepsy and in children with speech impairment and other disorders who do not have epileptic seizures, but epileptiform activity is detected according to the EEG (so-called conditions associated with benign epileptiform patterns of childhood (BEPD), cognitive epileptiform disintegration, etc.). Thus, in studies of the effectiveness and tolerability of the drug hopantenic acid in children with speech impairment and epileptiform activity of the DEPD type according to EEG data, in no case were negative dynamics recorded according to EEG results [11, 12]. V. I. Guzeva et al. (2015) used the drug hopantenic acid at a dose of 20–30 mg/kg/day in children aged 4 to 7 years with epilepsy and speech disorders. A study of speech function was carried out before the administration of the drug hopantenic acid and 2 months after the start of its administration. Nineteen children took the drug hopantenic acid in the form of syrup, 21 children - in the form of tablets (250 and 500 mg) during AED therapy. According to the results of this study, varying degrees of effectiveness of hopantenic acid were proven in 77.5% of children with epilepsy and speech disorders such as speech tempo disturbances, stuttering, an erased form of dysarthria, and general speech underdevelopment of mild to moderate severity. It is important to note that the use of the drug hopantenic acid did not lead to an increase in the index of epileptiform activity, while there was an improvement in the characteristics of the basic rhythm: there was an improvement in EEG indicators in the form of a decrease in diffuse changes and a delay in the maturation of the basic rhythm in 7.5% of children, in 7.5% children, EEG results began to correspond to the age norm. Thus, along with the effectiveness of hopantenic acid in the correction of speech disorders in children with epilepsy, its good tolerability and positive dynamics according to EEG data were noted. No complications from taking the drug or increased frequency of epileptic seizures were identified [9].
The advantage of hopantenic acid in the treatment of neurological disorders in children.
The advantage of hopantenic acid in childhood psychoneurology is due to its mild sedative effect in a certain (often individual) dose range and the relative rarity of the hyperstimulating effect, increasing the overall adaptive potential with expanding the range of adaptive capabilities by optimizing the somatovegetative, cognitive and emotional components of mental activity [18].
An important advantage is also the presence of official indications for use in the Russian Federation as a drug for the treatment of hyperkinetic disorder, which has a positive effect on excessive motor activity, impaired attention, impulsivity and comorbid conditions (enuresis, stuttering, anxiety disorders) [34–36].
The availability of a solution for oral administration of Gopantomide, in our opinion, is also convenient in the treatment of young children and in other cases when there are difficulties in taking the drug in tablet form.
Initially, the main indication for the use of hopantenic acid in children was urinary disorders (since 1980), which is associated with the ability of the drug to induce inhibition processes of the pathologically increased bladder reflex and detrusor tone. These indications (various neurogenic urinary disorders, including enuresis) are retained in the instructions for use of the drug to this day. However, subsequently the range of pathologies for the use of hopantenic acid preparations expanded significantly [33].
Taking into account the experience of previous studies of hopantenic acid drugs, currently the main directions of neuropediatrics and the main types of pathology of the nervous system for which Gopantomide can be used are the following:
perinatal neurology (perinatal damage to the nervous system and its outcomes, including cerebral palsy);
neurourology (enuresis, pollakiuria, urgency, imperative urinary incontinence);
cognitive neurology (delayed motor development, delayed speech development);
neurorehabilitation (consequences of traumatic brain injuries and neuroinfections);
epileptology (epilepsy with slowing of mental processes in complex therapy with anticonvulsants);
neuropsychiatry (neurotic disorders, behavioral disorders, ADHD), etc. [6, 7, 33].
However, in the practice of a neurologist, the main areas of application of Gopantomide currently remain in epileptology, in the treatment of developmental delay and correction of cognitive impairment of various etiologies, neurotic disorders and ADHD
Efficacy of hopantenic acid in the treatment of ADHD.
The effectiveness of hopantenic acid in the treatment of ADHD has been noted by many authors [10, 13, 16–18, 22]. The effectiveness of hopantenic acid in patients with ADHD can be explained by the nootropic profile of action, which helps to increase the psycho-emotional resources of adaptation, as well as the modulating effect on excessive motor activity and symptoms of impulsivity, which presumably contribute to the fixation of maladaptive patterns of behavioral response in case of long-term and persistent manifestations of maladjustment. Thus, the drug hopantenic acid can be effective for ADHD, including in combination with concomitant (comorbid) disorders that aggravate the clinical picture as a whole [22].
N. N. Zavadenko et al. (2017, 2018) conducted a multicenter, double-blind, placebo-controlled study of the use of the drug hopantenic acid in the pharmacotherapy of ADHD in children 6–12 years old [17, 18]. The drug hopantenic acid was prescribed in tablets of 250 mg at a therapeutic dose of 30 mg/kg of the child’s body weight, divided into 2 doses, therapy was carried out for 4 months. When assessing the patients' condition over time, the sum of scores on the ADHD-DSM-IV scale, the clinical general impression scale (CGI-ADHD-severity), functional impairment (WFIRS-P), and the results of the correction test were taken into account.
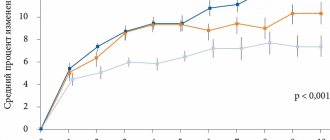
A detailed analysis of the interim results of this study involving 35 patients (18 received hopantenic acid (group 1) and 17 received placebo (group 2)) showed that after 4 months of treatment with hopantenic acid, the severity of ADHD symptoms on the scale was statistically significantly reduced Clinical Global Impression (CGI-ADHD-S). Initially, the severity of ADHD was assessed in the range from 4 (moderate) to 5 points (severe severity), but after 1 month of treatment with the drug hopantenic acid - in the range of 2–3 points (2 - minimal, 3 - mild), and remained at that level. same range after 2, 3 and 4 months of treatment. At the same time, the effectiveness of the drug hopantenic acid was manifested by a steady increase in the proportion of patients with a positive response to therapy (and a decrease in the total score on the ADHD-DSM-IV scale by more than 25%) from 44.4% after 1 month of treatment to 66.7% by the end of 2 months. ‑month of therapy and 72.2% - after the 3rd and 4th months. A significant regression of the main symptoms of ADHD on the ADHD-DSM-IV scale was observed after 1 month of treatment and continued until 2–4 months of treatment, accompanied by a decrease in all 3 assessments compared with the indicators before treatment (p < 0.01): total score, symptoms of “Attention disorders” and “Hyperactivity-impulsivity”. In addition, over 4 months of treatment with hopantenic acid, compared with placebo, the severity of functional impairment on the WFIRS-P scale decreased, especially in the “Child Self-Esteem” and “Risk Behavior” sections. Thus, reliable data have been obtained on the high therapeutic effectiveness of the drug hopantenic acid for ADHD. The therapy was well tolerated. Treatment with hopantenic acid for 4 months. at a therapeutic dose of 30 mg/kg was characterized by a favorable safety profile, which was not significantly different from placebo [17, 18].
The final analysis of the study results (in total, the study included 85 children with ADHD who were under outpatient supervision in 4 centers, of which 45 children received hopantenic acid (group 1) and 44 received placebo (group 2)) showed that the therapeutic effectiveness of the drug hopantenic acid for ADHD in children in comparison with placebo showed a pronounced tendency towards an increase in the proportion of patients with positive dynamics (a decrease in the total score on the ADHD-DSM-IV scale by more than 25%) by the end of the 3rd and 4th th months of therapy: response to therapy was achieved in 66.7 and 68.9% of patients, respectively, while in the placebo group - only in 52.3 and 61.4%. Also, when treated with hopantenic acid, the severity of the disease on the Clinical Global Impression scale was statistically significantly reduced compared to placebo. After 4 months of therapy, compared with placebo, the hopantenic acid drug significantly reduced the severity of functional impairment in 4 of 6 sections of the WFIRS-P scale: “Family,” “Education and School,” “Child Self-Esteem,” and “Risk Behavior.” Also, while taking the drug hopantenic acid, there was an improvement in the indicators of sustained attention in the correction test (quality and speed of execution) in children with ADHD compared to placebo.
Moreover, the drug had a favorable safety profile and did not differ from placebo in the frequency of adverse events [17, 18].
There is evidence of the successful use of hopantenic acid in combination with atomoxetine for ADHD in children, even in cases where atomoxetine alone was not effective enough.
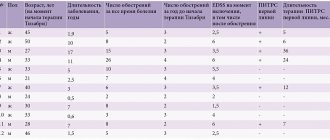
T. A. Kupriyanova et al. (2017) studied the effectiveness of the drug hopantenic acid in the adjunctive therapy of ADHD (according to the International Classification of Diseases, 10th revision (ICD-10) heading F90.1) in children with insufficient effectiveness of atomoxetine therapy. The authors conducted an open-label pilot non-randomized study involving 24 children (16 boys and 8 girls) aged 6 to 11 years (mean age 9.2 ± 2.8 years) with ADHD, 18 of whom were outpatients, 6 in the hospital. The average duration of the disease was 3.1 ± 1.8 years. The study included children with ADHD who had an insufficient effect from previous therapy with atomoxetine in adequate doses for at least 3 months. Efficacy was assessed using the Clinical Global Impression (CGI) scale and the adapted Children's Social Functioning Scales CGAS and CHIP-CE. With the additional administration of the drug hopantenic acid in combination with atomoxetine, a significant decrease in symptoms of hyperactivity and an increase in the level of social functioning and quality of life were noted. If at the initial assessment of ADHD according to ICD-10 criteria the severity of clinical manifestations averaged 24.0 ± 3.6 points, then after 1 month of therapy this figure was 18.0 ± 3.1 points, and by the end of the 2nd month - 15.0 ±2.9 points. The level of social functioning on the CGAS scale at the initial assessment was 41.2 ± 3.2 points, after 1 month of therapy - 50.5 ± 3.1, and by the end of the 2nd month - 54.7 ± 2.8 points. The CGI score at baseline averaged 3.8 ± 1.09 points, which was considered a moderate disorder. After 1 month, the score averaged 2.5 ± 0.17 points, and after 2 months - 1.9 ± 0.15, with a significant improvement noted in almost 70% of children, a less pronounced improvement in 25%, changes was not observed in 5% of children. These, as a rule, included older children, 10–11 years old, undergoing hospital treatment. Their ADHD was combined with severe comorbid disorders (affective, anxiety, tics, etc.). In addition, behavioral disorders in these children were more pronounced and resistant to treatment. According to the CHIP-CE scale, the most significant positive changes were noted in the domains of “achievement” (school performance) - from 23.6 ± 4.2 to 34.7 ± 3.8 points, “risk avoidance” - 26.8 ± 2.9 up to 37.8 ± 5.1 points. Less pronounced changes occurred in the domains “resistance” (“family involvement”) - from 22.7 ± 5.4 to 26.9 ± 3.6 points, “comfort” (emotional comfort) - from 21.8 ± 7.8 to 27.4 ± 2.7 points, “satisfaction” - from 24.5 ± 8.3 to 29.8 ± 5.2 points. However, positive changes were also noted in these domains. Qualitative changes related not only to achieving control over symptoms, but also to increasing the level of social functioning and quality of life of children show the effectiveness of hopantenic acid in this category of patients [22].
Efficacy of hopantenic acid in long-term treatment of ADHD.
ADHD symptoms require long-term treatment. According to N. N. Zavadenko et al. (2018), treatment of ADHD should be comprehensive, including both drug treatment and various types of behavioral, educational and neuropsychological correction, and family psychotherapy. At the same time, treatment of ADHD should be quite long-term and, in accordance with the modern concept of an expanded therapeutic approach, focused not only on reducing the main symptoms of ADHD, but also on overcoming dysfunction in various areas of the patient’s life, improving his adaptive capabilities and quality of life indicators [18] . As a study of a group of children with ADHD showed, during long-term courses of treatment with hopantenic acid, in order to overcome not only the main symptoms, but also adaptation disorders and socio-psychological functioning (improving self-esteem, communication with others and social activity), a treatment period of at least 4–6 months is required [13]. Thus, it is advisable to plan drug therapy for ADHD over several months, up to the duration of the entire school year [18]. This is due to the fact that, in contrast to regression of the main symptoms of ADHD, longer periods of therapy are required to overcome adaptation disorders and socio-psychological functioning. Significant improvements in self-esteem, communication with others and social activity were observed after 4 months, improvement in behavior and school performance, basic life skills, along with a significant regression in risk behavior - after 6 months of use of the drug hopantenic acid [18].
According to N. N. Zavadenko and N. Yu. Suvorinov (2011), hopantenic acid can be successfully used in long-term treatment of ADHD. At the same time, the positive effect of hopantenic acid on the main symptoms of ADHD was achieved after 2 months, and the positive effect continued to increase after 4–6 months of treatment [16].
The effectiveness of hopantenic acid in the correction of cognitive impairment in children.
In one of the first studies in clinical practice devoted to the correction of cognitive impairment of various etiologies in children using the drug hopantenic acid, O. I. Maslova et al. (2004) studied the effectiveness of the use of hopantenic acid in the form of syrup in the treatment of 59 children aged 7–8 years with various pathological conditions accompanied by functional impairment of cognitive functions [25]. The study included children with cephalgia, ADHD, vegetative-vascular dystonia, neurotic reactions, manifestations of asthenic syndrome against the background of high stress at school. Hopanthenic acid syrup was prescribed at a dose of 30–50 mg/kg/day for 2 oral doses, morning and afternoon, after meals. An examination was carried out using the Psychomat test computer system, and the basic parameters of cognitive functions were analyzed before and after a course of treatment with the drug hopantenic acid. A reliably significant positive effect of the drug on the studied parameters was demonstrated. In particular, in children with ADHD, while taking hopantenic acid syrup, the following improved: by 10–45% - indicators of psychomotor activity and hand-eye coordination; by 20–40% - indicators of short-term visual memory; by 30% - indicators of distribution and switching of attention - the basic function of analytical-synthetic processes. In general, the study by O.I. Maslova et al. [2004] demonstrated the effectiveness of hopantenic acid in the correction of cognitive impairment in children with ADHD, asthenoneurotic syndrome and cerebrasthenic syndrome due to traumatic brain injury [25].
Subsequently, a number of studies were carried out on representative populations of patients in various clinics, which confirmed the effectiveness of the drug hopantenic acid in the correction of cognitive functions in various pathologies in children. L.M. Kuzenkova et al. (2007, 2008) demonstrated the effectiveness of hopantenic acid in the correction of cognitive impairment in patients with enuresis [21], A. Yu. Tomilova et al. (2007) - for allergic rhinitis [37], and S. V. Balkanskaya et al. (2008) - in the complex therapy of Rolandic epilepsy [2–4]. According to O.I. Maslova (2006), the drug hopantenic acid is highly effective for the correction of cognitive disorders in children with increased educational loads during the school year [24]. Studies have been conducted confirming improved recovery after surgical treatment with the use of hopantenic acid in adults and children [27, 29]. M. V. Panteleeva et al. (2017) note the effectiveness of the drug hopantenic acid in the correction of postoperative cognitive dysfunction in children. According to the authors, in these cases it is advisable to administer the drug hopantenic acid at a dose of 40 mg/kg/day for 1 month after surgery [29].
The effectiveness of hopantenic acid in delayed speech and mental development in children.
N. N. Zavadenko and E. V. Kozlova (2013) conducted a comprehensive examination of 50 children aged 3 to 5 years with a developmental disorder of expressive speech (F80.1 according to ICD-10) and a picture of general speech underdevelopment of levels 1-2. As part of an open controlled study, children with developmental dysphasia were divided into 2 groups, which were observed for 2 months: Group 1 - 30 children (25 boys, 5 girls) who received the drug hopantenic acid; Group 2 (control) - 20 children (15 boys, 5 girls) who did not receive drug therapy. The drug hopantenic acid was prescribed in the form of syrup at a daily dose of 500–600 mg (30–35 mg/kg) for two months in monotherapy in 2 doses - in the morning (after breakfast) and in the afternoon (after naps and afternoon snacks). To reduce the likelihood of side effects, the dose was gradually increased in the first 7–10 days of use. Before the start of the treatment course (day 0) and at the end of the course (day 60), children with dysphasia underwent a neurological, psychological and speech therapy examination. In the group of patients who received a course of treatment with the drug hopantenic acid, a significant improvement was achieved on all scales studied: expressive, impressive speech and speech attention. In contrast to the control group, children receiving the drug hopantenic acid showed a significant improvement in all analyzed indicators: there was a significant increase in the number of spoken words (active vocabulary), the average and maximum number of syllables in spoken words, the number of phrases in colloquial speech, the average and maximum number of words in phrases. In the control group, only an increase in the volume of the active vocabulary and the number of phrases was noted. However, during treatment with the drug hopantenic acid, these indicators increased more than 3 times, and in the control group - only 1.5 times. The results obtained, according to the authors of the study, allow us to conclude that nootropic drugs hopantenic acid have a significant positive effect on the speech state of children with developmental dysphasia with general speech underdevelopment of levels 1-2. As a survey of the patients' parents showed, during treatment with the drug hopantenic acid, there was a significant decrease in the severity of cerebrasthenic symptoms (fatigue, emotional instability, tearfulness, poor appetite, headaches, difficulty falling asleep, restless shallow sleep), psychosomatic disorders (unreasonable pain in the abdomen and other parts of the body). body, enuresis, parasomnias), motor awkwardness and fine motor difficulties. At the same time, attention characteristics improved, regression of hyperactivity, emotional-volitional disorders (behavior inappropriate for age, shyness, fear of not being liked by others, excessive touchiness, inability to stand up for oneself, feeling unhappy), behavior problems, aggressiveness and oppositional behavior was observed. On the contrary, in the control group there was only a slight decrease in psychosomatic disorders and anxiety. Since children with developmental dysphasia often have impaired fine motor skills, clumsiness, clumsiness, and poor coordination of movements, the authors included methods for studying the motor sphere with a score for completing tasks for walking along a line, maintaining balance, the level of formation of motor skills (jumping, playing with a ball). ). The data obtained coincided with the results of a questionnaire survey of the patients' parents and indicated a positive effect of the drug hopantenic acid on the motor sphere of children with developmental dysphasia, movement coordination and the formation of motor skills.
The authors noted the low frequency and insignificant severity of side effects of the drug hopantenic acid [14].
The effectiveness of hopantenic acid in the correction of speech disorders in combination with epileptiform activity according to the morphology of the DEPD according to EEG data in children.
N. N. Zavadenko et al. (2014) considered the possibilities of therapeutic correction of speech disorders in combination with DEPD based on EEG data in children. The authors focused on the category of pediatric patients with specific speech development disorders or developmental dysphasia. With these disorders, speech suffers already in the early stages of a child’s development without a previous period of normal development, and their prevalence in the child population reaches 5–10%. In patients with developmental dysphasia, epileptiform changes according to EEG data have been described, including the so-called DEPD, which is explained in the concept of congenital disorder of brain maturation developed by H. Doose et al. According to this concept, some patients have a genetically determined disorder of brain maturation in the prenatal period, which is the cause of a complex of pathological conditions: epileptic seizures, DEPD-type patterns according to EEG data, and developmental disorders, in particular dysphasia and autistic disorders. According to EEG data, the appearance of epileptiform activity of the DEPD type is usually observed between the ages of 3 and 6 years. When conducting EEG or EEG monitoring C. Duvelleroy-Hommet et al. (1995) identified DEPD in 38% of 24 children with developmental dysphasia, A. Picard (1998) - in 50% of 52.L. Neuschlova et al. (2007) - in 39% of 28 patients. According to N. N. Zavadenko et al. (2014), to overcome speech disorders, children with developmental dysphasia are shown complex therapy along with speech therapy and psychological and pedagogical correction, including repeated courses of nootropic drugs. When developmental dysphasia is combined with subclinical epileptiform activity according to EEG data, preference should be given to nootropic drugs that do not cause an increase in the epileptiform activity index. Such a drug is hopantenic acid; the positive effect of this drug has been confirmed not only in developmental dysphasia in children, but also in the treatment of speech, cognitive and behavioral disorders in a group of patients with epilepsy aged 3–4 years, among whom there were no negative dynamics were recorded according to EEG data [10, 11, 12, 14].
The effectiveness of hopantenic acid in the treatment of asthenic syndrome in children.
Hopantenic acid preparations can be effective in the treatment of asthenic syndrome in adults and children [20, 24, 38].
In one of the earlier works by O.I. Maslova (2006), the drug hopantenic acid was highly effective for the correction of cognitive disorders in children with increased educational loads during the school year [24].
According to a recent study by L. S. Chutko et al. (2018), these data are confirmed. The authors studied the use of hopantenic acid in the treatment of asthenic syndrome (functional asthenia, increased fatigue) in children of primary school age. The study included 50 children aged 7 to 9 years with functional asthenia. The control group consisted of 30 healthy children aged 7–9 years. Children in the study group took the drug hopantenic acid at a dose of 750 mg/day for 4 weeks. To assess the dynamics of the main symptoms, the subjective asthenia rating scale (MFI-20), visual analogue scale (10-point version), and psychophysiological test TOVA were used. The results of the study showed the high effectiveness of hopantenic acid (improvement was obtained in 72% of cases). After treatment, asthenic symptoms significantly decreased, and there was an improvement in attention and reaction time. Adverse events (hyperexcitability, difficulty falling asleep) while taking hopantenic acid were observed in 3 (6%) cases; they were moderate in nature, did not cause refusal of further therapy and disappeared after the end of the treatment course. The authors concluded that hopantenic acid is an effective treatment for functional asthenia in children [38].
According to researchers, the drug hopantenic acid can be used in preventive medicine, as a drug to prevent the formation of neurotic disorders. N.K. Sukhotina et al. (2004) conducted studies with the participation of children representing a risk group for the development of neurotic disorders. The authors revealed a decrease in the level of situational anxiety, especially manifested in the situation of assessing their competence; they showed a decrease in emotional lability, touchiness, irritability of children, and a more even mood background [34–36].
Efficacy of hopantenic acid in the treatment of neuroinfections in children.
N.V. Skripchenko et al. (2019) noted a good effect of using hopantenic acid in the complex therapy of tick-borne encephalitis in children. In combination with etiotropic therapy, patients of all groups received hopantenic acid as part of neurometabolic therapy, which also included cytoflavin, choline alfoscerate, Actovegin, and neurovitamins [30].
The effectiveness of hopantenic acid in the treatment of tics in children.
V.P. Zykov notes the effectiveness of hopantenic acid in the treatment of tics and Tourette's syndrome. According to the author, in children aged 5–8 years, it is advisable to start initial therapy with hopantenic acid at a dose of 50 mg/kg/day, twice a day, for a course of 4–6 months; after 1 month of use, along with regression of tics, a decrease in the level of anxiety and severity of ADHD manifestations is observed [19].
Pantocalcin tablet. 250mg N50
Registration Certificate Holder
VALENTA PHARMACEUTICS (Russia)
Dosage form
Medicine - Pantocalcin® (Pantocalcin)
Description
Pills
white, flat-cylindrical in shape, with a chamfer and a notch.
1 tab.
calcium hopantenate (hopantenic acid or calcium salt of hopantenic acid) 250 mg
Excipients
: magnesium hydroxycarbonate - 46.77 mg, calcium stearate - 3.1 mg, talc - 62 mg, potato starch - 3.93 mg.
10 pieces. — contour cellular packaging (5) — cardboard packs. 50 pcs. — polymer jars (1) — cardboard packs.
Indications
Cerebrovascular insufficiency caused by atherosclerotic changes in cerebral vessels, senile dementia (initial forms), residual organic brain damage in mature and elderly people, cerebral organic insufficiency in patients with schizophrenia, extrapyramidal hyperkinesis in patients with hereditary diseases of the nervous system (including Huntington's chorea, hepatocerebral dystrophy, Parkinson's disease), residual effects of previous neuroinfections, post-vaccination encephalitis, traumatic brain injury (as part of complex therapy); extrapyramidal neuroleptic syndrome (hyperkinetic and akinetic), as a corrector of the side effects of antipsychotics (neuroleptics) and for preventive purposes at the same time as “cover therapy”; epilepsy (with slow mental processes in combination with anticonvulsants). Psycho-emotional overload, decreased mental and physical performance (increased concentration and memory). Urinary disorders: enuresis, daytime urinary incontinence, pollakiuria, urgency (adults and children over 2 years old).
Children: perinatal encephalopathy, mental retardation (delayed mental, speech, motor development or a combination thereof), cerebral palsy, stuttering (mainly clonic form), epilepsy (as part of combination therapy with anticonvulsants, especially with polymorphic seizures and petit mal seizures) .
Contraindications for use
Acute severe kidney disease, first trimester of pregnancy.
pharmachologic effect
A nootropic agent that has neurometabolic, neuroprotective and neurotrophic properties. Increases the brain's resistance to hypoxia and the effects of toxic substances, stimulates anabolic processes in neurons, combines a moderate sedative effect with a mild stimulating effect, has an anticonvulsant effect, reduces motor excitability while regulating behavior. Increases mental and physical performance. Helps normalize GABA content during chronic alcohol intoxication and subsequent ethanol withdrawal. Shows analgesic effect.
Drug interactions
Prolongs the effect of barbiturates, enhances the effects of anticonvulsants, nootropics and central nervous system stimulants, and the effect of local anesthetics (procaine).
Prevents side effects of phenobarbital, carbamazepine, antipsychotics (neuroleptics).
The effect of hopantenic acid is enhanced in combination with glycine and xydiphone.
Dosage regimen
Taken orally. Single dose for adults - 0.5-1 g, for children - 0.25-0.5 g; daily dose for adults - 1.5-3 g, for children - 0.75-3 g. The course of treatment is 1-4 months, in some cases - up to 6 months. After 3-6 months, a second course of treatment is possible.
For children with mental impairment and mental retardation - 0.5 g 4-6 times a day daily for 3 months; for delayed speech development - 0.5 g 3-4 times a day for 2-3 months.
As a corrector for neuroleptic syndrome, adults - 0.5-1 g 3 times/day, children - 0.25-0.5 g 3-4 times/day. The course of treatment is 1-3 months.
For epilepsy, children - 0.25-0.5 g 3-4 times a day, adults - 0.5-1 g 3-4 times a day, daily, for a long time (up to 6 months).
For tics in adults - 1.5-3 g/day, daily, for 1-5 months; children - 0.25-0.5 g 3-6 times a day daily for 1-4 months.
For urinary problems in adults - 0.5-1 g 2-3 times a day, daily dose - 2-3 g; for children, a single dose is 0.25-0.5 g, daily - 25-50 mg/kg. The course of treatment is 0.5-3 months.
Side effect
Allergic reactions:
rhinitis, conjunctivitis, skin rashes.
special instructions
Long-term therapy with hopantenic acid in combination with other nootropic and central nervous system stimulants is not recommended.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated.
Use is contraindicated in the first trimester of pregnancy.
Use for renal impairment
Restrictions for impaired renal function - Contraindicated.
The drug is contraindicated in acute severe kidney disease.
Use in children
Restrictions for children - No restrictions.
Application is possible according to the dosage regimen.