Pharmacodynamics
Pimecrolimus is a derivative of the macrolactam ascomycin and has an anti-inflammatory effect. Pimecrolimus selectively inhibits the production and release of cytokines and inflammatory mediators from T lymphocytes and mast cells.
Pimecrolimus specifically binds to the cytosolic receptor macrophilin-12 and inhibits the calcium-dependent phosphatase calcineurin. Inhibition of calcineurin leads to suppression of T-lymphocyte proliferation and prevents the transcription and production of early cytokines in T-helper types 1 and 2, such as IL-2, interferon-γ, IL-4, IL-5, IL-10, tumor necrosis factor (TNFα) and granulocyte-macrophage colony-stimulating factor. Pimecrolimus and tacrolimus equally suppress the secondary immune response in isolated skin T helper cell colonies obtained from patients with atopic dermatitis.
In addition, in vitro, after interaction with the antigen/IgE complex, pimecrolimus prevents the antigen/IgE-mediated release of cytokines and inflammatory mediators from mast cells. Pimecrolimus does not affect the growth of keratinocytes, fibroblasts and endothelial cells and, unlike corticosteroids, has a selective effect on cells of the immune system and does not cause dysfunction, viability, differentiation processes, maturation of Langerhans cells in mice and dendritic cells of monocytic origin in humans. The drug does not affect the differentiation of “naive” T-lymphocytes into T-effector cells under the influence of Langerhans cells and dendritic cells, which is one of the main mechanisms of a specific immune response.
In experimental models of skin inflammation, the high anti-inflammatory activity of pimecrolimus was demonstrated after its local and systemic application. When applied topically in experimental models of allergic contact dermatitis (ACD), pimecrolimus is comparable in effectiveness to highly active corticosteroids: clobetasol-17-propionate and fluticasone, inhibits the inflammatory response in response to skin irritants, without causing changes in skin consistency and atrophy.
In addition, when administered topically and orally, pimecrolimus effectively reduces skin inflammation, itching, and the severity of histopathological changes in experimental models of ACD. When applied topically, the penetration of tacrolimus and pimecrolimus into the skin is equally good. However, the ability of pimecrolimus to penetrate the skin is less than that of tacrolimus and GCS. Thus, pimecrolimus has a selective effect on the skin.
The unique mechanism of action of pimecrolimus is the combination of a selective anti-inflammatory effect on the skin with a slight effect on the systemic immune response.
When used for 6 weeks in children aged 3 months to 17 years, pimecrolimus effectively reduces itching and skin inflammation (erythema, infiltration, excoriation and lichenification). With long-term use for 12 months, pimecrolimus effectively reduces the incidence of sudden exacerbations of ACD without causing atrophy, irritation or increased sensitivity of the skin, and without phototoxic or photosensitizing effects.
Elidel in dermatological practice
IN AND. Kulagin, D.K. Nazhmutdinova, E.V. Taha
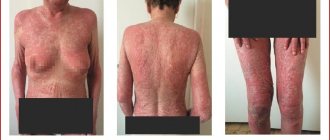
Atopic dermatitis (AD) is one of the most pressing problems of dermatology, which is associated with a significant increase in the incidence of this dermatosis, characterized by a chronic course with frequent relapses, as well as the insufficient effectiveness of existing treatment methods. The incidence of AD has increased in recent decades. Among people born before 1960, 1.4% to 3% had one or more episodes of BP, and among those born after 1970, the rate ranged from 8.9% to 20.4%. The incidence of AD is especially high in children.
In developed countries, approximately 10–15% of children under 5 years of age suffer from AD, and 48–75% of them show initial signs of the disease within the first 6 months. life. Thus, AD has become a socially significant disease, especially given the high incidence among children. Clinical manifestations of atopic dermatitis are characterized by intense itching, inflammation, infiltration, lichenification in typical locations of the skin process, as well as general increased dryness of the skin. The disease most often occurs in childhood; relapses are usually associated with errors in diet and stress. Atopic dermatitis is an immune-dependent disease characterized by the immune system by hyperproduction of IgE, disruption of cytokine regulation and the Th1/|Th2 lymphocyte ratio, deterministic deficiency of suppressor T lymphocytes, and disruption of apoptosis processes. In the pathogenesis of the disease, an imbalance of intracellular regulatory mechanisms (cAMP/cGMP ratio), disruption of membrane reception, activation of non-immune mechanisms for the release of allergic mediators, disruption of neurovegetative and peripheral circulation with vascular instability and disruption of endothelial reception are essential; psychophysiological and psychosomatic abnormalities. In the external treatment of this dermatosis, corticosteroids are often used, which are not free from side effects. However, significant progress and scientific achievements in the field of clinical immunology have made it possible to decipher many previously unknown mechanisms of the immunopathogenesis of this disease, which served as an incentive for the creation of new drugs with targeted, selective effects on allergic processes in the skin.
These drugs are external inhibitors of inflammatory cytokines, belonging to the class of ascomycin macrolactams. On the domestic drug market, a representative of this group is 1% pimecrolimus (Elidel cream, Switzerland). Pimecrolimus (Elidel) is a new nonsteroidal inflammatory cytokine inhibitor developed specifically as a topical treatment for atopic dermatitis. This substance has a selective effect on the inflammatory process in the skin and does not affect the local and systemic immune response. Pimecrolimus is a derivative of the macrolactam ascomycin. Selectively inhibits the production and release of cytokines and mediators from T lymphocytes and mast cells. Has anti-inflammatory properties. Pimecrolimus specifically binds to macrophilin-12 and inhibits the calcium-dependent phosphatase calcineurin. As a result, by blocking the transcription of early cytokines, pimecrolimus suppresses the activation of T lymphocytes. In particular, in nanomolar concentrations, pimecrolimus inhibits the synthesis of interleukin-2, interferon-? in human T-lymphocytes. (Th1 type), interleukin-4 and interleukin-10 (Th2 type). In addition, in vitro, after interaction with the antigen/IgE complex, pimecrolimus prevents the antigen/IgE-mediated release of cytokines and inflammatory mediators from mast cells. Pimecrolimus does not affect the growth of keratinocytes, fibroblasts and endothelial cells. In experimental models of skin inflammation, the high anti-inflammatory activity of pimecrolimus was demonstrated after its local and systemic application. When applied topically in porcine models of allergic contact dermatitis (ACD), pimecrolimus is as effective as the highly active corticosteroids clobetasol-17-propionate and fluticasone.
Unlike clobetasol-17-propionate, pimecrolimus does not cause skin atrophy in pigs. Unlike clobetasol-17-propionate and fluticasone, pimecrolimus also does not cause thickening or changes in skin consistency in pigs. Pimecrolimus has been shown to inhibit the inflammatory response to cutaneous irritants in rat models of contact dermatitis. In addition, when administered topically and orally, pimecrolimus effectively reduces skin inflammation and pruritus and normalizes histopathological changes in hypomagnesemic hairless rats, which are models of acute atopic dermatitis. Compared to tacrolimus, topical pimecrolimus penetrates the skin equally well in experimental models. However, due to its high lipophilicity, the penetration of pimecrolimus through the skin is 10 times less than that of tacrolimus. Therefore, pimecrolimus has a selective effect on the skin. Pimecrolimus is effective against skin inflammation, but at the same time its effect on the systemic immune response is very small. Overall, the unique mechanism of action of pimecrolimus is the combination of skin-selective anti-inflammatory activity with a low ability to induce systemic immune responses. Treatment should begin at the first manifestations of the disease to prevent its sudden exacerbation. 1% Elidel cream 2 times a day is applied in a thin layer to the affected surface and gently rubbed until completely absorbed.
The drug can be applied to the skin of any part of the body, including the head, face, neck, as well as to diaper rash areas. The cream should be applied 2 times a day until symptoms disappear completely. After cessation of treatment, in order to avoid subsequent exacerbations, at the first signs of relapse of atopic dermatitis, therapy should be resumed. Emollients can be applied immediately after applying Elidel 1% cream. However, after water procedures, emollients should be used before applying Elidel cream. The use of Elidel 1% cream may cause minor transient reactions at the application site, such as a feeling of warmth and/or burning. If these reactions are severe, patients should consult a doctor. The most commonly reported side effects were application site reactions, which occurred in 19% of patients treated with Elidel 1% cream and 16% of control patients. These reactions mainly occurred early in treatment and were minor/moderate and short-lived.
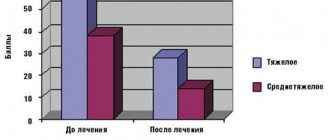
Frequency of side effects: very often? 10%; often – from?1% to <10%; sometimes – from ?0.1% to <1%; rarely – from ?0.01% to <0.1%; very rare <0.01%. Very common: burning sensation at the site of application of the cream. Common: local reactions (irritation, itching and redness of the skin), skin infections (folliculitis). Sometimes: suppuration; worsening of the disease; herpes simplex; dermatitis caused by the herpes simplex virus (eczema herpes); molluscum contagiosum, local reactions such as rash, pain, paresthesia, peeling, dryness, swelling, skin papillomas, boils. Clinical studies of Elidel (E) in the treatment of patients with AD demonstrated a rapid onset of action of the drug, in which the intensity of itching decreased after 2-3 days of using Elidel cream, and all symptoms of AD regressed in most patients during the course of therapy. The beneficial effect of treatment with pimecrolimus was observed throughout the study (up to 43 days) in infants 3–23 months and children 2–17 years old, and E was especially effective when the process was localized on the face and neck, and during subsequent clinical follow-up, the absence of pronounced AD symptoms persisted for up to 6 months [Eichenfield L. et al., 2002; HoV. et al., 2003]. Subsequent clinical studies confirmed the effectiveness of the use of E in the treatment of AD in children and adults, and established that long-term (up to 6 months) use of E helps control the disease and prevents the development of severe exacerbations of AD, while at the same time does not lead to the development of significant side effects, such as pyogenic or viral superinfection. Clinical studies conducted in Russia (Russian State Medical University, Moscow Medical Academy named after I.M. Sechenov, Ural Research Institute of Dermatovenerology and Immunopathology, etc.), with the participation of patients with AD of various age groups, in the treatment of which the new topical drug Elidel cream was used, demonstrated effectiveness of more than 95% of patients.
The effect of the drug was noted already in the first week of use, when the area of skin lesions and the severity of AD symptoms decreased by 1.4–1.8 times, and the intensity of itching and sleep disturbances decreased by almost 2 times. The SCORAD score decreased by 2.5–4.6 times compared to the baseline. Elidel cream was especially clinically effective in the treatment of patients with AD in children under 3 years of age and adolescents, which was confirmed by a significant decrease in the SCORAD index and its components. In adult patients, a significant decrease in the intensity of itching was noted after completion of therapy. During the study, there were no adverse events or complications; Elidel cream was well tolerated in most patients. Thus, summarizing the results of research and the experience of foreign and domestic scientists, we can conclude that Elidel cream is effective in the treatment of children, adolescents, and adults with AD, and its use in widespread clinical practice by dermatologists allows optimizing the treatment of patients with AD.
Literature
1. Atopic dermatitis in children. Guide for doctors. Edited by N.G. Korotky. 2003; 82–90.
2. Atopic dermatitis. Guide for doctors. Ed. Yu.V. Sergeeva. 2002; 8 p.
3. Balabolkin I.I., Grebenyuk V.N. Atopic dermatitis in children, – M.: Medicine, 1999. – 238 p.
Kungurov N.V., Gerasimova N.M., Kokhan M.M. Atopic dermatitis (types of course, principles of therapy) - Ekaterinburg: Ural Publishing House. Univ., 2000.–266 p.
4. Kungurov N.V., Kokhan M.M.Keniksfest Yu.V. et al. Experience of using Elidel cream in the treatment of topical dermatitis in children and adults: Breast Cancer, v. 12, no. 4, pp. 171–174. Sergeev Yu.V., Ivanov O.L., Novikov D.K. Atopic dermatitis: modern diagnosis and treatment // Immunopathology, allergology, infectology. –2001. –No. 4. -WITH. 28–48
5. Skripkin Yu.K., Samsonov V.A., Selissky G.D., Gomberg M.A. Modern problems of dermatovenerology // Bulletin of dermatology and veterinary
neurology. –1997. –N6. –P.4–8.
6. Smirnova G.I. Modern technologies for local treatment of atopic dermatitis in children // Immunopathology, allergology, infectology. –2003. -No. 3. -WITH. 75–82.
7. Fedenko E. S. Atopic dermatitis: rationale for a step-by-step approach to therapy // Consilium medicum. –2001. –No. 3 (4). -WITH. 176–184.
8. Hultsch T. et al. Ascomycin macrolactam derivative SDZ ASM 981 inhibits the release of granule–associated mediators and of newly synthesized cytokines in RBL 2H3 mast cells in an immunophilin–dependent manner // Arch. Dermatol. Res. – 1998. –Vol.290. –P.501–507.
9. Kapp A. et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug // J. Allergy Clin. Immunol. – 2002. –Vol.110. –P.277–284.
10. Lewis–Jones MS, Finlay AY, Dykes PJ The Infants' Dermatitis Quality of Life Index // Br. J. Dermatol. – 2001. –Vol. 144. –P. 104–110.
11. Meurer M. et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: A six-month study // Dermatology. – 2002. –Vol. 205. –P.271–277.
12. Schultz–Larsen F, Hanifin JM. Epidemiology of atopic dermatitis // Immunol. Allergy Clin. North Am. –2002. –Vol.22. –P.1–24.
13. Stuetz A et al. Pimecrolimus does not affect Langerhans' cells in murine epidermis, in contract to corticosteroids // J. Invest. Dermatol. – 2002. –Vol.119. P. 347
14. Tofte SJ, Hanifin J..M. Current management and therapy of atopic dermatitis // J. American Acad. Dermatol. –2001. –Vol. 44(1). -R. 13–16.
15. Wahn U et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children // Pediatrics. – 2002. – Vol. 110. –P.158–159.
Published with permission from the administration of the Russian Medical Journal.
Pharmacokinetics
Adults. The concentration of pimecrolimus in the blood was determined in 12 adult patients with atopic dermatitis (eczema) affecting 15–59% of the body surface area, treated with Elidel cream 2 times a day for 3 weeks. In 77.5% of observations, the concentration of pimecrolimus in the blood was below 0.5 ng/ml (minimum detectable concentration), and in 99.8% it was below 1 ng/ml. Cmax of pimecrolimus in the blood, recorded in 1 patient, was 1.4 ng/ml.
In 98% of 40 adult patients with initial lesions of 14–62% of the body surface area, after 1 year of treatment with Elidel cream, pimecrolimus blood concentrations remained low and in most cases were below the minimum detectable concentration.
A Cmax value of 0.8 ng/ml was recorded after 6 weeks of treatment in only 2 patients. None of the patients showed an increase in concentration over 12 months of treatment. During a 3-week period of treatment with Elidel cream twice daily in 13 adult patients with hand dermatitis (using the cream on the palms and dorsum of the hands and bandaging at night), the maximum recorded blood concentration of pimecrolimus was 0.91 ng/ml.
In 8 patients with pimecrolimus blood levels above the minimum detectable concentration, the AUC value was 2.5–11.4 ng/ml.
Children. Pharmacokinetic studies of pimecrolimus were conducted in 58 children aged 3 months to 14 years with atopic dermatitis (eczema) affecting 10–92% of the body surface area, treated with Elidel cream 2 times a day for 3 weeks. Five children received treatment for 1 year as needed.
Pimecrolimus blood concentrations were consistently low, regardless of the area of skin lesions and duration of therapy, and were in the same range as in adult patients receiving Elidel cream therapy at the same doses. In 97% of cases, pimecrolimus blood concentrations were below 2 ng/ml, and in 60% they were below 0.5 ng/ml (minimum detectable concentration). The Cmax of pimecrolimus recorded in 2 patients aged 8 months and 14 years was 2 ng/ml.
Among the youngest children (from 3 to 23 months), the Cmax of pimecrolimus was 2.6 ng/ml and was recorded in 1 patient.
In 5 children treated with Elidel cream for 1 year, pimecrolimus concentrations were at consistently low levels. The maximum value recorded in 1 child was 1.94 ng/ml. Throughout the entire treatment period, an increase in drug concentrations was not observed in any of the patients.
In 8 children aged 2 to 14 years with blood levels of pimecrolimus above the minimum detectable concentration when measured three times, the AUC value ranged from 5.4–18.8 ng/ml. AUC values in patients with skin lesions less than or greater than 40% were comparable.
In in vitro studies, the binding of pimecrolimus to plasma proteins (mainly various lipoproteins) was 99.6%.
Since the concentrations of pimecrolimus in the blood are very low when applied topically, determination of metabolic parameters is not possible.
Pharmacokinetics in special clinical situations
Atopic dermatitis (eczema) is rarely observed in patients aged 65 years and older. The number of patients of this age in clinical studies of Elidel 15% cream was insufficient to detect any difference in treatment effectiveness compared with younger patients.
Dosing recommendations for infants (3–23 months), children (2–11 years), and adolescents (12–17 years) are the same as for adult patients.
Contraindications
hypersensitivity to timecrolimus or any components of the drug;
children under 3 months of age (the safety and effectiveness of Elidel cream in children under 3 months of age has not been studied);
the presence of acute viral, bacterial or fungal skin infections.
Carefully:
patients with Netherton's syndrome (no safety data available) - there may be a risk of increased systemic absorption of the drug;
severe forms of inflammation or skin damage, incl. generalized erythroderma (no data on safety of use) - there may be a risk of increased systemic absorption of the drug;
weakened immunity - because the effectiveness and safety of use have not been studied.
There are no data on the safety of long-term use of Elidel cream.
Since the effect of long-term use of the drug on the immune defense of the skin and the incidence of malignant neoplasms has not been studied, Elidel cream should not be applied to damaged areas of the skin with possible malignancy or dysplastic changes.
In case of bacterial or fungal infection of the skin, the use of Elidel cream on the affected areas is possible only after the infection has been cured.
Use during pregnancy and breastfeeding
There are no data on the use of the drug in pregnant women. In experimental studies with local use of the drug, no direct or indirect damaging effect of Elidel cream on the course of pregnancy, the development of the embryo/fetus, the course of childbirth and the postnatal development of the offspring was not revealed. Caution should be exercised when prescribing to pregnant women. However, given the minimal absorption of pimecrolimus when administered topically, the potential risk in humans is considered negligible.
The excretion of the drug into breast milk after topical administration has not been studied in experimental models. There are no data on the content of pimecrolimus in the breast milk of lactating women.
Since many drugs are excreted in breast milk, caution should be exercised when prescribing Elidel 1% cream to nursing women. However, given the minimal systemic absorption of pimecrolimus when administered topically, the potential risk to humans is considered negligible.
Breastfeeding women should not apply Elidel 1% cream to the breast area.
The effect of Elidel cream on fertility in men and women has not been established.
Pharmacological properties of the drug Elidel
Pimecrolimus is a derivative of the macrolactam ascomycin. Selectively inhibits the production and release of cytokines and mediators from T lymphocytes and mast cells. Has anti-inflammatory properties. Pimecrolimus binds highly specifically to macrophilin-12 and inhibits the calcium-dependent phosphatase calcineurin. As a result, by blocking the transcription of early cytokines, pimecrolimus suppresses the activation of T lymphocytes. In particular, in nanomolar concentrations, pimecrolimus inhibits the synthesis of interleukin-2, interferon gamma (Th1 type), interleukin-4 and interleukin-10 (Th2 type) in human T lymphocytes. In addition, in vitro after interaction with the antigen/IgE-pimecrolimus complex, it prevents the antigen/IgE-mediated release of cytokines and inflammatory mediators from mast cells. Pimecrolimus does not affect the growth of keratinocytes, fibroblasts and endothelial cells. The drug combines high anti-inflammatory activity and a slight effect on systemic immune responses. When pimecrolimus is applied topically, its concentration in the blood is very low, so it is impossible to determine metabolic parameters. In vitro in human skin .
Side effects
The use of Elidel cream may cause minor transient reactions at the application site, such as a feeling of warmth and/or burning. If these reactions are severe, patients should consult a doctor.
The most common reactions at the site of application of the drug were observed in 19% of patients treated with Elidel cream and in 16% of patients in the control group. These reactions mainly occurred early in treatment and were minor/moderate and short-lived.
Determination of the frequency of adverse reactions: very often (≥1/10); often (≥1/100, <1/10); sometimes (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (<1/10000), including isolated reports.
Very often - burning at the site of application of the cream.
Often - local reactions (irritation, itching and redness of the skin), skin infections (folliculitis).
Sometimes - suppuration, worsening of the disease, herpes simplex, dermatitis caused by the herpes simplex virus (herpetic eczema), molluscum contagiosum; local reactions such as rash, pain, paresthesia, peeling, dryness, swelling, skin papillomas, boils.
The following adverse reactions were observed during post-marketing use of the drug (frequency estimate based on the number of cases of adverse events in an unspecified population).
From the immune system: very rarely - anaphylactic reactions.
Metabolic disorders (metabolic disorders): rarely - alcohol intolerance.
From the skin and its appendages: rarely - allergic reactions (rash, urticaria, angioedema); changes in skin color (hypopigmentation, hyperpigmentation).
In most cases, facial redness, rash, burning, itching or swelling developed immediately after drinking alcohol.
When using Elidel cream, in rare cases, the development of malignant neoplasms, including skin and other types of lymphomas, and skin cancer was observed. A cause-and-effect relationship between these adverse events and the use of the drug has not been established.
Side effects of the drug Elidel
Common: burning sensation at the site of application of the cream, reactions at the site of application (irritation, rash, erythema), skin infections (folliculitis). Rarely: impetigo, deterioration of the condition, herpes simplex and herpes zoster, herpes dermatitis, Kaposi's varioliform pustulosis, molluscum contagiosum, disturbance at the application site - pain, paresthesia, peeling, dryness, swelling, skin papilloma, boil, alcohol intolerance (in most such cases after When drinking alcohol, there were sensations of blood flow, rash, itching or swelling), allergic reactions (skin rash, urticaria, angioedema) and changes in skin color (hypo- or hyperpigmentation). Very rare : anaphylactic reactions. In isolated cases, malignancies, including cutaneous and other types of lymphomas, as well as skin cancer, have been reported in patients using pimecrolimus cream, although a causal relationship has not been established.
Interaction
The potential interaction of Elidel cream with other drugs has not been studied. Given that the systemic absorption of pimecrolimus is very low, any interaction of Elidel cream with drugs for systemic use is unlikely.
When using Elidel cream in children aged 2 years and older, the drug did not affect the effectiveness of vaccination.
It is not recommended to apply cream to the area where the vaccine was administered until local manifestations of the post-vaccination reaction have completely disappeared.
Incompatibility. Since compatibility studies have not been conducted, it is not recommended to use the drug in combination with other topical agents.
Directions for use and doses
Externally.
Treatment should begin at the first manifestations of the disease to prevent its sudden exacerbation.
The cream is applied in a thin layer to the affected surface 2 times a day and gently rubbed until completely absorbed.
The cream can be applied to the skin of any part of the body, including the head, face, neck, as well as to diaper rash areas. Elidel cream should be used 2 times a day until the symptoms of the disease completely disappear. If the severity of symptoms persists, after 6 weeks of using the drug, it is necessary to re-examine the patient to confirm the diagnosis of atopic dermatitis. After cessation of treatment, in order to avoid subsequent exacerbations, at the first signs of relapse of atopic dermatitis, therapy should be resumed. Emollients can be applied immediately after applying Elidel 1% cream. However, after water procedures, emollients should be used before applying Elidel cream.
Given the very low systemic absorption of pimecrolimus, there are no restrictions on the total daily dose of the drug applied, the area of skin surface treated, or the duration of treatment. If Elidel cream gets into your eyes or mucous membranes (oral or nasal cavity), you should immediately remove the cream and rinse your eyes and mucous membranes with running water.
special instructions
When treated with topical calcineurin inhibitors, including Elidel, the development of malignant neoplasms (for example, skin tumors and lymphomas) has been observed in rare cases. A cause-and-effect relationship between these adverse events and the use of the drug has not been established.
In clinical studies when using Elidel cream, 0.9% of patients (14 out of 1544) experienced the development of lymphadenopathy. Typically, lymphadenopathy was caused by infectious diseases and disappeared after a course of appropriate antibiotic therapy. In all patients, it was either possible to identify the cause of the development of lymphadenopathy, or the disappearance of this undesirable phenomenon was noted. In patients receiving treatment with Elidel, if lymphadenopathy develops, it is necessary to establish the etiology of the process and monitor patients until this adverse event completely disappears. If the etiology of lymphadenopathy is unknown or if the patient has acute mononuclear inflammation, the drug should be discontinued.
When treated with Elidel cream, patients are advised to reduce artificial or natural insolation of the skin to a minimum or completely eliminate UV irradiation. The possible effect of using the drug on skin lesions caused by UV radiation is unknown.
Influence on the ability to drive vehicles and operate machinery. The effect of using Elidel cream on the ability to drive vehicles or operate machinery has not been established.
Special instructions for the use of Elidel
Should not be applied to skin affected by acute viral infection. In case of bacterial or fungal skin infection, the cream can be used after the infection has been cured. If the infection process does not decrease, the use of the cream should be discontinued until the symptoms of the infection are relieved. Since the effect of Elidel 1% cream upon long-term use on local immunity and on the manifestations of malignant skin neoplasms is not known, it should not be used in cases of potentially malignant skin neoplasms or the possibility of such diseases. Although a causal relationship has not been established, there have been rare cases of skin malignancies and lymphoma in patients treated with topical calcineurin inhibitors, including Elidel 1% cream. This medicinal product is not recommended for patients with Netherton's syndrome or generalized erythroderma, where there is a risk of increased absorption, since the safety of Elidel 1% cream in patients with these diseases has not been established. The safety and effectiveness of Elidel 1% cream in patients with immunodeficiency have also not been studied, therefore the use of the drug in this group of patients is not recommended. In clinical studies of Elidel 1% cream, 0.9% of cases of lymphadenopathy were noted. Typically, they were associated with infections and disappeared with appropriate antibiotic therapy (most of them had a known etiology or disappeared on their own). Therefore, when lymphadenopathy appears in patients who used Elidel 1% cream, it is necessary to clarify the etiology of this process. If the etiology of lymphadenopathy is unclear or if acute infectious mononucleosis occurs, treatment with the drug should be discontinued. It is necessary to monitor the dynamics of patients with symptoms of emerging lymphadenopathy to monitor its cure. During treatment with Elidel 1% cream, it is advisable for patients to limit exposure to natural or artificial sunlight as much as possible or avoid it altogether, even when the cream is not applied to the affected areas of the skin. The potential effect of Elidel 1% cream on affected skin that is exposed to ultraviolet radiation is not known. Cases of atopic dermatitis (eczema) are rare in patients aged 65 years and older. Clinical studies studying Elidel 1% cream did not include a sufficient number of patients in this age category to determine whether their response to the drug differs from that of younger patients. The use of the cream may cause minor transient reactions at the site of application, such as a sensation of warmth and/or burning. If these reactions are severe, patients should consult a doctor. The cream should not be applied to mucous membranes. If the drug accidentally gets on the mucous membrane or in the eyes, rinse them immediately with water. During pregnancy and breastfeeding. There is no data on the use of Elidel cream during pregnancy. Caution should be exercised when prescribing Elidel 1% cream during this period. It is unknown whether pimecrolimus passes into breast milk, so caution should be exercised when prescribing Elidel 1% cream during breastfeeding. During breastfeeding, mothers should not apply 1% Elidel cream to the breast area. Effect on the ability to drive vehicles and operate machinery: not established.