Curiosin gel for external use approx 0.103% 15g aluminum tube.
Registration Certificate Holder
GEDEON RICHTER (Hungary)
Dosage form
Medicine - Curiosin®
Description
Gel for external use
colorless or almost colorless, transparent, practically odorless.
1 g
1 tube
zinc hyaluronate* 1.03 mg 15.4 mg
* obtained according to prescription: sodium hyaluronate - 15 mg, zinc chloride - 5 mg.
Excipients
: potassium sorbate, sodium hydroxide, carbomer, water d/i.
15 g - aluminum tubes (1) - cardboard packs.
Indications
- local treatment of all forms of mild to moderate acne, primarily comedones and papulopustular acne.
Contraindications for use
- hypersensitivity to the components of the drug;
- poultry protein intolerance;
- pregnancy;
- breastfeeding period.
pharmachologic effect
Zinc hyaluronate combines the wound-healing and stimulating effects of zinc and hyaluronic acid. The main component of Curiosin®, hyaluronic acid, is an essential component of the skin and connective tissue, ensuring the integrity of its structure, elasticity and regeneration after damage. Hyaluronic acid is one of the most biocompatible materials for wound care.
Zinc hyaluronate forms an extracellular matrix, accelerating granulation, angiogenesis and epithelization, resulting in increased synthesis and accumulation of collagen fibers. Thanks to its antimicrobial properties, zinc prevents the development of infection. Being an antioxidant, it also prevents damage to the cellular and extracellular matrix by free radicals, reducing their negative effect on tissue regeneration.
Zinc hyaluronate accelerates the healing of damaged skin by primary or secondary intention, creating a special microenvironment in the damaged area as a result of the retention and delivery of water, acting as a mechanical and antimicrobial barrier and forming a biocompatible molecular protective layer, which leads to relief of pain in the wound area and suppression of the inflammatory process . The hydrated layer of zinc hyaluronate forms a dense molecular network that acts as a viscoelastic molecular filter and prevents the penetration of microorganisms from the external environment (airborne and contact). Thus, the drug serves as a kind of hydrated antimicrobial dressing, keeping the damaged skin in a hydrated state, which is the main purpose of managing skin lesions.
Healing may be accompanied by a weakening of scarring (fetal wounds heal without scarring due to the extremely high content of hyaluronic acid) with the restoration of damaged skin to an almost healthy state and appearance.
Compared to sodium hyaluronate, zinc hyaluronate has a bacteriostatic effect against many bacteria.
In particular, zinc inhibits the activity of Propionibacterium acnes
.
Release of biologically active substances in response to the activity of Propionibacterium acnes
is an important factor in addition to the two main causes of acne development (proliferation of tissues inside the sebaceous glands and hyperkeratosis of their ducts). These processes lead to a significant decrease in the concentration of hyaluronic acid in the affected tissues. The lack of hyaluronic acid can be compensated for by topical application of the drug Curiosin® gel. Curiosin® gel softens the consequences of the development of comedones, as well as inflammation.
Drug interactions
There are no data on interactions with other drugs.
Dosage regimen
Externally. The gel is applied 2 times a day to the affected areas of the skin in a thin layer after preliminary cleansing. The drug is absorbed within a few minutes and does not require the use of a bandage. The duration of therapy is individual and depends on the form and severity of the disease.
Overdose
Cases of overdose of the drug Curiosin® gel have not been registered.
Side effect
The following adverse reactions are listed according to MedDRA according to frequency of occurrence: uncommon (≥0.01%, <1%);
very rare (<0.1%, including isolated cases). From the skin and subcutaneous tissues:
uncommon* - redness, tingling at the site of application, burning sensation, local pain; very rarely** - excessive development of granulation tissue, necrosis in the wound.
*adverse reactions develop at the beginning of treatment, usually mild and transient.
** connection with the drug Curiosin® gel has not been established.
Other:
the development of allergic reactions is possible. When applied to dry skin, a “tightening” feeling may occur.
special instructions
The drug does not cause photosensitivity and does not stain skin or underwear.
The tip of the tube should not come into contact with the skin or any other surface. When applying, precautions should be taken to prevent microbial contamination or deterioration of the product.
One tube should only be used by one patient.
Before first use, make sure that the protective membrane is intact. The tube should be carefully closed after each use. Effect on the ability to drive vehicles and machinery.
Does not affect the ability to drive vehicles and machinery.
Storage conditions
The drug should be stored out of the reach of children at a temperature of 15° to 30°C.
Best before date
Shelf life: 2 years. Do not use after the expiration date stated on the package.
Use during pregnancy and breastfeeding
There is no data on the use of the drug during pregnancy and lactation.
Terms of sale
The drug is available without a prescription.
Contacts for inquiries
GEDEON RICHTER JSC (Hungary)
Organization accepting complaints from consumers Moscow representative office of JSC Gedeon Richter 119049 Moscow, 4th Dobryninsky lane, 8 Tel. Email
Experience with the use of curiosin for the treatment of erosive and ulcerative skin lesions
Skin lesions that occur with a violation of the integrity of the skin and the formation of superficial (erosions) or deep (ulcers) defects can be observed in many diseases. The development of these morphological elements can be caused by numerous factors: local ischemia (Martorell's ulcer, neurotrophic ulcers, etc.), infectious and inflammatory processes (pyoderma, tuberculosis), trauma (pathomimia), including minor ones, when erosions develop at friction sites , pressure, when eating solid foods (hereditary pemphigus (epidermolysis bullosa), etc.
The nature, features of the course and prognosis are determined by the nosological form of the disease. A common factor that worsens both the course and the prognosis for any pathological process accompanied by the formation of erosive and ulcerative elements is the addition of a secondary infection, which requires the appointment, in addition to special (pathogenetic) drugs (for example, corticosteroids and immunosuppressants for pemphigus, systemic vasculitis; vasodilators drugs for trophic ulcers; antibiotics for pyoderma, etc.), and drugs aimed at stimulating the healing of erosions and ulcers, local and systemic antibacterial drugs. However, the latter have a number of side effects, in particular, an immunosuppressive effect, which negatively affects the processes of epithelization of erosive and ulcerative lesions.
Currently, there is an extremely limited number of drugs that have both antibacterial and regenerative effects. Therefore, the purpose of our study was to study the clinical effectiveness of the drug Curiosin in the treatment of erosive and ulcerative skin defects, including infected ones.
Curiosin
is a combination of hyaluronic acid and zinc. Thanks to the hyaluronic acid included in the drug, the drug stimulates the process of epithelization of erosive and ulcerative lesions. Zinc, widely used in dermatology as part of various external agents, has an antimicrobial and anti-inflammatory effect, which makes it possible to limit the use of local anti-inflammatory and antibacterial drugs, which, as a rule, inhibit the healing process.
The effectiveness of Curiosin has been studied in diseases such as epidermolysis bullosa
(simple localized and generalized, dystrophic autosomal dominant and autosomal recessive),
true pemphigus, chancriform pyoderma, ecthyma, ulcerative vegetative pyoderma, ulcerative necrotizing vasculitis
.
Previous studies in epidermolysis bullosa have examined the effectiveness of Curiosin gel. In this study, we used Curiosin in solution form. Moreover, the effectiveness of the solution for this pathology was comparable to the effectiveness of the gel form of the drug.
Of 18 patients with epidermolysis bullosa
a simple localized form was observed in 9 patients, a simple generalized form - in 2 patients, dystrophic autosomal dominant epidermolysis bullosa - in 4 patients, dystrophic autosomal recessive - in 3 patients. Curiosin was prescribed 2 times a day at the rate of 1 drop per 1 cm2 of surface (in the autosomal recessive form - under a bandage). In simple and dominant dystrophic forms of the disease, epithelization of erosive foci occurred on average on the 3rd day; in the autosomal recessive form, the period of epithelization was 5-6 days.
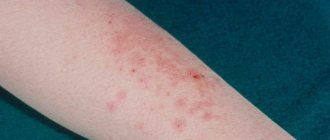
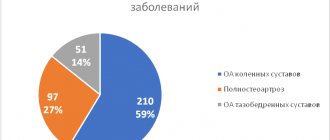
With true pemphigus
complete epithelization of erosions was observed on the 5th day of therapy, while on the background of placebo the elements healed on average in 3 weeks (Fig. 1).
When prescribing Curiosin to 6 patients with superficial ecthyma
relief of inflammation and suppression of the infectious process was noted on days 3-4, complete healing occurred on day 7 of treatment.
Chancriform pyoderma
was observed in 4 patients, while in one of them the ulcerative area did not heal within six months against the background of traditionally used antibacterial and epithelialization-stimulating therapy. Patients were prescribed Curiosin at the rate of 1 drop per 1 cm2. Healing of ulcerative defects was observed on days 20-21 of treatment.
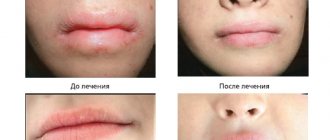
On foci of ulceration that have developed in place of pustular elements in ulcerative-vegetative pyoderma
Curiosin solution was used 2 times a day, also at the rate of 1 drop per 1 cm2. The surface of the lesion was pre-treated with saline solution. Against the background of the applied therapy with Curiosin, it was noted (Fig. 2) the disappearance of discharge from the lesion on average on the 4th day, the beginning of granulation and scarring of the lesion from the periphery - on the 6-8th day, complete healing of the ulcer with the formation of a smooth scar - on the 14th day.
Clinical results, almost identical to those with ulcerative-vegetative pyoderma, were obtained in the treatment of patients with ulcerative-necrotizing vasculitis
. Thus, a decrease in exudative manifestations was noted on average on the 2-3rd day of therapy, the beginning of the process of granulation and scarring of the lesion from the periphery - on 6-8 days, a reduction in the lesion by half - on average on the 12th day, and its scarring - on the 16th day (Fig. .3).
None of the patients using Curiosin experienced any side effects; in all cases, the drug was well tolerated. Considering the above, it is advisable to use Curiosin solution for these diseases. This drug undoubtedly has significantly greater potential for use in dermatological practice, which should be the subject of further study.
Zinc hyaluronate –
Curiosin (trade name)
(GEDEON RICHTER)
| Applications to the article |
| Figure 1. Effectiveness of curiosin in true pemphigus |
| Figure 2. Efficacy of curiosin in ulcerative-vegetative pyoderma |
| Figure 3. Efficacy of curiosin in ulcerative-necrotizing vasculitis |