Pharmacological properties of the drug Tamiflu
Mechanism of action. Antiviral drug. Oseltamivir phosphate is a prodrug, its active metabolite (oseltamivir carboxylate) is an effective and selective inhibitor of neuraminidase of influenza viruses type A and B - an enzyme that catalyzes the process of release of newly formed viral particles from infected cells, their penetration into respiratory epithelial cells and further spread of the virus in the body . Inhibits the growth of the influenza virus in vitro and suppresses the replication of the virus and its pathogenicity in vivo, reduces the release of influenza A and B viruses from the body. Tamiflu significantly shortens the period of clinical manifestations of influenza infection, reduces their severity and reduces the incidence of influenza complications requiring the use of antibiotics (bronchitis, pneumonia, sinusitis, otitis media), shortens the time of virus isolation from the body and reduces the area under the “viral titer-time” curve . In children aged 1 to 12 years, Tamiflu significantly reduces the duration of the disease (by 35.8 hours) and the incidence of acute otitis media. Recovery and return to normal activity occurs almost 2 days earlier. When taken for the purpose of prevention, Tamiflu significantly (by 92%) reduces the incidence of influenza among contacted persons, by 76% - the frequency of clinically established influenza during an outbreak of the disease, reduces the frequency of virus isolation and prevents the transmission of the virus from one family member to another. In children aged 1 to 12 years, preventive use of Tamiflu reduces the incidence of laboratory-confirmed influenza from 24 to 4%. Tamiflu does not affect the formation of anti-influenza antibodies, incl. to produce antibodies in response to the administration of an inactivated influenza vaccine. Resistance. When taking Tamiflu for the purpose of prophylaxis (7 days), prophylaxis of family contacts (10 days) and seasonal prophylaxis (42 days), no cases of drug resistance were observed. In adult patients/adolescents, oseltamivir resistance was detected in 0.32% of cases (4/1245) using phenotyping and in 0.4% of cases (5/1245) using phenotyping and genotyping, and in children from 1 year to 12 years in 4.1% (19/464) and 5.4% (25/464) of cases, respectively. All patients had temporary carriage of an OS-resistant virus. This did not affect virus clearance. Several different subtype-specific viral neuraminidase mutations have been discovered. The degree of sensitivity reduction depended on the type of mutation; for example, with the 1222V mutation in N1, sensitivity decreased by 2 times, and with R292K in N2, by 30,000 times. No mutations were found to reduce the sensitivity of influenza B virus neuraminidase in vitro. In patients treated with oseltamivir, reported neuraminidase N1 mutations (including H5N1 viruses) leading to resistance/reduced susceptibility to OS were H274Y, N294S (1 case), E119V (1 case), R292K (1 case), and neuraminidase mutations N2 - N294S (1 case) and SASG245-248del (1 case). In one case, the G402S mutation of the influenza B virus was detected, which resulted in a 4-fold decrease in sensitivity, and in 1 case, the D198N mutation was detected with a 10-fold decrease in sensitivity in a child with immunodeficiency. Viruses with a resistant neuraminidase genotype differ in resistance to varying degrees from the natural strain. Viruses with the R292K mutation in N2 in animals (mice and ferrets) are significantly inferior in infectivity, pathogenicity and contagiousness to viruses with the E119V mutation in N2 and D198N in B and differ slightly from the natural strain. Viruses with the H274Y mutation in N1 and N294S in N2 occupy an intermediate position. Pharmacokinetics Oseltamivir phosphate is easily absorbed from the gastrointestinal tract and is highly converted into an active metabolite under the action of hepatic and intestinal esterases. Concentrations of the active metabolite in plasma are determined within 30 minutes, the time to reach Cmax is 2–3 hours, and are more than 20 times higher than the concentrations of the prodrug. At least 75% of the dose taken orally enters the systemic circulation in the form of an active metabolite, less than 5% in the form of the parent drug. Plasma concentrations of both the prodrug and the active metabolite are dose proportional and independent of food intake. The volume of distribution (Vss) of the active metabolite is 23 l. After oral administration of oseltamivir phosphate, its active metabolite was detected in the lungs, bronchial lavage waters, nasal mucosa, middle ear and trachea in concentrations providing an antiviral effect. Binding of the active metabolite to plasma proteins is 3%. Prodrug binding to plasma proteins is 42%, which is not enough to cause significant drug interactions. Oseltamivir phosphate is highly converted into an active metabolite under the action of esterases located mainly in the liver and intestines. Neither oseltamivir phosphate nor the active metabolite are substrates or inhibitors of cytochrome P450 isoenzymes. It is excreted (>90%) as an active metabolite mainly by the kidneys. The active metabolite does not undergo further transformation and is excreted in the urine (>99%) by glomerular filtration and tubular secretion. Renal clearance (18.8 l/h) exceeds the glomerular filtration rate (7.5 l/h), indicating that the drug is also eliminated by tubular secretion. Less than 20% of the drug taken is excreted in feces. T1/2 of the active metabolite - 6-10 hours. Pharmacokinetics in special groups Patients with kidney damage. When Tamiflu is prescribed to patients with varying degrees of kidney damage, AUC values are inversely proportional to the decrease in renal function. Patients with liver damage. In vitro, in patients with hepatic pathology, neither a significant increase in the AUC of oseltamivir phosphate nor a decrease in the AUC of the active metabolite was observed. Elderly patients. In elderly patients (65–78 years), the exposure to the active metabolite at steady state is 25–35% higher than in younger patients when similar doses of Tamiflu are prescribed. T1/2 of the drug in elderly patients did not differ significantly from that in younger patients. Elderly patients do not require dose adjustment for the treatment and prevention of influenza. Children. In young children, clearance of the prodrug and active metabolite occurs more rapidly than in adults, resulting in lower AUCs relative to a given dose. Taking the drug at a dose of 2 mg/kg provides the same AUC of oseltamivir carboxylate as is achieved in adults after a single dose of a capsule with 75 mg of the drug (equivalent to approximately 1 mg/kg). The pharmacokinetics of oseltamivir in children over 12 years of age is the same as in adults.
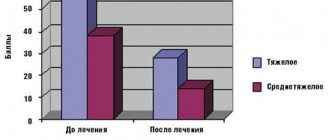
Therapeutic effect
Neuraminidase is an enzyme found on the surface of the virus particle. Thanks to it and another protein (hemagglutinin), the virus penetrates into a healthy cell. The enzyme destroys the cell membrane. The viral particle enters the bloodstream and, with the help of neuraminidase, acts on sialic acids located on the surface of red blood cells.
In a cell, pathogenic microorganisms replicate using its resources. Neuraminidase promotes the release of new viral particles from the infected cell. It can accelerate the penetration of microbes through the mucous barrier of the respiratory system.
When drugs have an inhibitory effect on neuraminidase, the ability of viral particles to penetrate into the cell is impaired, and the process of excretion of new virions is also disrupted. This leads to limiting the spread of infection through the respiratory tract.
Neuraminidase inhibitors are used in the treatment of influenza. They are most effective if treatment began in the first two days after the onset of clinical symptoms of the disease.
Respiratory signs of influenza:
- Cough.
- Nasal congestion, runny nose.
- Sore and sore throat.
Symptoms of general intoxication of the body with influenza:
- High body temperature (above 38 °C).
- Headache.
- weakness.
- Chills.
- Muscle pain, body aches.
- Nausea, vomiting.
- Decreased appetite.
Most often, patients with suspected influenza seek medical help only on the second or third day after the onset of the disease. Taking the drug can alleviate the course of the disease. Patients taking Oseltamivir had a reduced risk of complications.
Neuraminidase inhibitors and other antiviral drugs should not be used to treat colds that are not caused by a virus.
Unreasonable use of medications provokes the emergence of resistance of pathogenic microorganisms to drugs. To avoid the spread of resistant strains of the influenza virus, drugs containing oseltamivir are rarely used for prophylactic purposes. According to some clinical recommendations, it is advisable to use Oseltamivir in combination with other antiviral medications (interferon inducers), which will increase the effectiveness of treatment.
Use of the drug Tamiflu
During preclinical studies, oseltamivir and its active metabolite were excreted into the milk of lactating rats. Whether oseltamivir or the active metabolite is excreted in human milk is unknown, but their amounts in breast milk may be 0.01 and 0.3 mg/day, respectively. Since there is insufficient data on the use of the drug in pregnant women, Tamiflu should be prescribed during pregnancy or nursing mothers only if the possible benefits of its use outweigh the potential risk to the fetus or infant. Inside, during meals or regardless of meals. Tolerability of the drug can be improved if taken with food. Preparation of the suspension:
- Gently tap the closed bottle with your finger several times so that the powder is distributed at the bottom of the bottle.
- Measure 52 ml of water using a measuring cup, filling it to the indicated level.
- Add 52 ml of water to the bottle, cap and shake well for 15 seconds.
- Remove the cap and insert the adapter into the neck of the bottle.
- Screw the cap onto the bottle tightly to ensure proper placement of the adapter.
The expiration date of the prepared suspension should be indicated on the bottle label. Before use, the bottle with the prepared suspension must be shaken. To dose the suspension, a dosing syringe is supplied with labels indicating dose levels of 30, 45 and 60 mg (see "Form and Packaging"). In cases where adults, adolescents ≥12 years of age and children weighing >40 kg or ≥8 years have problems swallowing capsules and Tamiflu powder for the preparation of oral suspension is not available, or if there are signs of “aging” of the capsules, it is necessary open the capsule and pour the contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food (chocolate syrup (normal or sugar-free), honey, light brown sugar or table sugar dissolved in water, sweet dessert , sweetened condensed milk, applesauce or yogurt) to cover up the bitter taste. The mixture must be mixed thoroughly and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Detailed recommendations are given below (see “Extemporaneous preparation of Tamiflu suspension”). Standard dosage regimen Treatment The drug should be started no later than 2 days from the development of symptoms of the disease. Adults and adolescents ≥12 years 75 mg (capsules or suspension) 2 times a day orally for 5 days. Increasing the dose to more than 150 mg/day does not increase the effect. Children >40 kg or ≥8 years Children who can swallow capsules can also be treated by taking 1 capsule. 75 mg 2 times a day, as an alternative to the recommended dose of Tamiflu suspension (see below). Children ≥1 year
Table 1 Recommended dosage regimen for Tamiflu oral suspension
Weight, kg | Recommended dose for 5 days (2 times a day, mg) |
| ≤15 | 30 |
| >15-23 | 45 |
| >23-40 | 60 |
| >40 | 75 |
To dose the suspension, use the supplied syringe marked 30, 45 and 60 mg. Take the required amount of suspension from the bottle with a dosing syringe, transfer it to a measuring cup and take orally.
Prevention Adults and adolescents ≥12 years old 75 mg 1 time per day for at least 10 days after contact with an infected person. The drug should be taken no later than in the first 2 days after contact. During a seasonal flu epidemic - 75 mg 1 time per day for 6 weeks. The preventive effect lasts as long as the drug is taken. Children >40 kg Children who can swallow capsules can also receive preventive therapy by taking 1 capsule. 75 mg once daily as an alternative to the recommended dose of Tamiflu suspension (see below). Children ≥1 year
Table 2 Recommended dosage regimen for Tamiflu suspension for oral administration
Weight, kg | Recommended dose for 10 days (once daily, mg) |
| ≤15 | 30 |
| >15-23 | 45 |
| >23-40 | 60 |
| >40 | 75 |
To dose the suspension, use the supplied syringe marked 30, 45 and 60 mg. Take the required amount of suspension from the bottle with a dosing syringe, transfer it to a measuring cup and take orally.
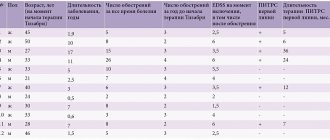
Dosing in special cases Patients with kidney damage Treatment. Patients with creatinine Cl >30 ml/min do not require dose adjustment. In patients with creatinine Cl from 10 to 30 ml/min, the dose of Tamiflu should be reduced to 75 mg once a day for 5 days. There are no dosing recommendations for patients on chronic hemodialysis or chronic peritoneal dialysis for end-stage chronic renal failure and for patients with creatinine Cl ≤10 ml/min. Prevention. Patients with creatinine Cl >30 ml/min do not require dose adjustment. In patients with creatinine Cl from 10 to 30 ml/min, it is recommended to reduce the dose of Tamiflu to 75 mg every other day or 30 mg suspension daily. There are no dosing recommendations for patients on chronic hemodialysis or chronic peritoneal dialysis for end-stage chronic renal failure and for patients with creatinine Cl ≤10 ml/min. Patients with liver damage. No dose adjustment is required for the treatment and prevention of influenza in patients with mild to moderate liver dysfunction. The safety and pharmacokinetics of Tamiflu in patients with severe hepatic impairment have not been studied. Elderly patients. No dose adjustment is required for the prevention or treatment of influenza. Children. The safety and effectiveness of Tamiflu in children under 1 year of age have not been established. Extemporaneous preparation of Tamiflu suspension In cases where adults, adolescents and children have a problem with swallowing capsules, and the powder for preparing the Tamiflu oral suspension is not available or if there are signs of “aging” of the capsules, it is necessary to open the capsule and pour its contents into a small amount ( maximum 1 teaspoon) of a suitable sweetened food item (see above) to cover the bitter taste. The mixture must be mixed thoroughly and allowed to be taken completely by the patient. The mixture should be taken immediately after preparation. If patients require a dose of 75 mg, the following instructions should be followed:
- Carefully open one 75 mg Tamiflu capsule over a small container and pour the powder into the container.
- Add a small amount (no more than 1 teaspoon) of a suitable sweetened food (to cover the bitter taste) and mix well.
- Mix the mixture thoroughly and give the entire contents of the container to the patient. The mixture should be taken immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and give the patient the remaining mixture to drink.
If patients require doses of 30–60 mg, the following instructions should be followed for proper dosing:
- Carefully open one 75 mg Tamiflu capsule over a small container and pour the powder into the container.
- Add 5 ml of water to the powder using a syringe with marks indicating the amount of liquid collected. Mix thoroughly for 2 minutes.
- Draw the required amount of mixture from the cup into the syringe according to Table 3. There is no need to withdraw undissolved white powder, since it is an inactive filler. By pressing the plunger of the syringe, inject all its contents into the second container. Any remaining unused mixture should be discarded.
- In a second container, add a small amount (no more than 1 teaspoon) of a suitable sweetened food to cover the bitter taste and mix well.
- Mix the mixture thoroughly and give the entire contents of the second container to the patient. The mixture should be taken immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and give the patient the remaining mixture to drink.
Repeat this procedure before each dose of the drug.
Table 3 Recommended dosage regimen for Tamiflu suspension for oral extemporaneous preparation
Weight, kg | Recommended dose, mg | Amount of mixture per dose, ml |
| ≤15 | 30 | 2 |
| >15-23 | 45 | 3 |
| >23-40 | 60 | 4 |
Application of Turkish medicine and dosage
Taking the drug does not eliminate the need for vaccination.
For children under one year of age, the medication is prescribed after a doctor has assessed their condition and weighed the risks. The dosage for small patients depends on body weight and is therefore calculated individually. The drug should not be used to treat premature babies born before the thirty-seventh week of pregnancy.
To prevent influenza, the medication is taken according to the following regimens:
- After close contact with carriers of a viral infection, the recommended dose of Enfluvir 75 mg Sert Kapsul (capsule drug) is 75 mg per day orally. This is one capsule. It should be washed down with water. The prophylactic course lasts ten days. Treatment should begin within the first two days after close contact with infected people.
- The recommended dosage for prevention during an influenza epidemic is 75 mg (capsules or tablets) per day. The optimal duration of preventive measures is six weeks.
- Children over one year of age can take 75 mg of Enfluvir once daily. The course is ten days. For babies under one year old, 30 mg per day is recommended. The course is also ten days. It is forbidden to give antiviral drugs to children without consulting a pediatrician.
- The prophylactic dose for a child under one year of age is selected by the doctor based on the baby’s weight. It is calculated individually. Typically, the amount of the drug is 2.5 mg or 3 mg per kilogram of body weight.
- During an influenza outbreak, the recommended prophylactic dose for children under one year of age is slightly different. It is half of the therapeutic daily norm.
Before taking Influvir, you need to read the instructions for Enfluvir 75 mg in Russian. In addition, you should consult your doctor
Side effects of Tamiflu
Adults. The most common are nausea and vomiting (usually after taking the first dose; they are transient in nature and in most cases do not require discontinuation of the drug). Side effects (≥1%): diarrhea, bronchitis, abdominal pain, dizziness, headache, cough, sleep disturbances, weakness; pain of various localizations, rhinorrhea, dyspepsia and upper respiratory tract infections. Children. The most common thing is vomiting. Abdominal pain, nosebleeds, hearing impairment, conjunctivitis (occurred suddenly, stopped despite continued treatment, and in the vast majority of cases did not cause cessation of treatment), nausea, diarrhea, asthma (including exacerbation), acute otitis media , pneumonia, sinusitis, bronchitis, dermatitis, lymphadenopathy. Post-marketing surveillance From the skin and subcutaneous tissue: rarely - hypersensitivity reactions: dermatitis, skin rash, eczema, urticaria, very rarely - erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis, anaphylactic and anaphylactoid reactions, Quincke's edema. From the liver: very rarely - hepatitis, increased liver enzymes. Neuropsychiatric: Convulsions and delirium have been reported in patients (mainly children and adolescents) taking Tamiflu to treat influenza (including symptoms such as impaired consciousness, disorientation in time and space, abnormal behavior, delirium, hallucinations, agitation, anxiety, nightmares). These cases rarely involved life-threatening actions. The role of Tamiflu in the development of these phenomena is unknown. Similar neuropsychiatric disorders were also noted in patients with influenza who did not receive Tamiflu. From the gastrointestinal tract: cases of gastrointestinal bleeding were rarely observed during treatment with Tamiflu (in particular, a connection between the phenomena cannot be excluded; they disappeared both after the patient recovered from the flu, as well as after discontinuation of the drug).
Patient opinions
Reviews about drugs containing oseltamivir are mostly positive. People write that the medicine very quickly helps normalize body temperature and relieves other flu symptoms. Side effects are rare. Most often these are nausea, headache, weakness, and decreased attention.
The disadvantages include the cost of the drugs. For example, the price of Oseltamivir is from 1000 rubles. Tamiflu costs from 1,300 rubles. for 10 capsules. Enfluvir 75 mg can be purchased at a price starting from RUB 1,380. per package.
Special instructions for the use of Tamiflu
Seizures and delirium-like neuropsychiatric disorders have been reported in patients (mostly children and adolescents) taking Tamiflu to treat influenza. These cases involved life-threatening actions. The role of Tamiflu in the development of these phenomena is unknown. Similar neuropsychiatric disorders were also noted in patients with influenza who did not receive Tamiflu. Careful monitoring of the behavior of patients, especially children and adolescents, is recommended to identify signs of abnormal behavior. There is no data on the effectiveness of Tamiflu for any diseases caused by pathogens other than influenza A and B viruses. When treating and preventing influenza in patients with creatinine Cl from 10 to 30 ml/min, dose adjustment is required. There are no recommendations for dose adjustment in patients receiving hemodialysis or peritoneal dialysis and in patients with creatinine Cl ≤10 ml/min. A 30 g bottle of Tamiflu with powder for preparing a suspension contains 25.713 g of sorbitol. When taking 45 mg of Tamiflu 2 times a day, 2.6 g of sorbitol enters the body. In patients with congenital fructose intolerance, this amount exceeds the daily norm of sorbitol. After preparation, store the suspension at a temperature of 2–8 °C for 17 days or at a temperature not exceeding 25 °C for 10 days. Do not use the suspension after the expiration date.
Who should not use the Turkish medicine "Influvir"
According to the instructions for Enfluvir 75 mg in Russian, the drug is not prescribed if the patient has the following diseases and conditions:
- Kidney failure. The standard dosage for influenza treatment is 75 mg twice daily. Dose adjustment is carried out taking into account the severity of kidney disease. The drug is not recommended for patients on dialysis.
- Liver failure. For mild or moderate liver dysfunction, the patient can take the standard therapeutic dose. In case of severe liver failure, it is recommended to refrain from using the drug.
- Influvir is not prescribed to children under one year of age without urgent need.
- Breast-feeding. Influvir passes into breast milk. Therefore, lactation should be stopped while taking it.
Tamiflu drug interactions
Clinically significant drug interactions are unlikely. Drug interactions due to competition and binding to the active sites of esterases that convert oseltamivir phosphate into the active substance are not presented. The low degree of binding of oseltamivir and the active metabolite to proteins does not give reason to assume the presence of interactions associated with the displacement of drugs from binding to proteins. In vitro, oseltamivir phosphate and the active metabolite are not the preferred substrate for polyfunctional oxidases of the cytochrome P450 system or for glucuronyltransferases (see “Pharmacokinetics”). There are no reasons for interaction with oral contraceptives. Cimetidine, a nonspecific inhibitor of cytochrome P450 isoenzymes, amoxicillin and paracetamol do not affect plasma concentrations of oseltamivir and its active metabolite. Probenecid leads to an approximately 2-fold increase in the AUC of the active metabolite of oseltamivir, but no dose adjustment is required when used concomitantly with probenecid. When Tamiflu is prescribed together with ACE inhibitors (enalapril, captopril), thiazide diuretics (bendrofluazide), antibiotics (penicillin, cephalosporins, azithromycin, erythromycin and doxycycline), histamine H2 receptor blockers (ranitidine, cimetidine), beta-blockers (propranolol) no changes in the nature or frequency of adverse events were observed.