Pharmacological properties of the drug Insulin detemir
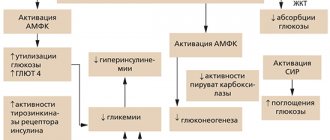
Modern recombinant DNA technologies have made it possible to improve the action profile of simple (regular) insulin. Insulin detemir is produced by recombinant DNA biotechnology using the Saccharomyces cerevisiae , and is a soluble basal analogue of long-acting human insulin with a peak-free action profile. The action profile is significantly less variable compared to isophane insulin and insulin glargine. The prolonged action is due to the pronounced self-association of insulin detemir molecules at the injection site and the binding of molecules to albumin through connection with the side fatty acid chain. Insulin detemir, compared to isophane insulin, is distributed more slowly in peripheral target tissues. These combined delayed distribution mechanisms provide a more reproducible absorption and action profile of insulin detemir. Insulin detemir is characterized by significantly greater intra-individual predictability of action in patients compared to NPH insulin or insulin glargine. This predictability of action is due to two factors: insulin detemir remains in a dissolved state at all stages from its dosage form to binding to the insulin receptor and the buffering effect of binding to serum albumin. By interacting with a specific receptor on the outer cytoplasmic membrane of cells, it forms an insulin-receptor complex that stimulates intracellular processes, including the synthesis of a number of key enzymes (hexokinase, pyruvate kinase, glycogen synthetase, etc.). The decrease in glucose content in the blood is due to an increase in its intracellular transport, increased uptake by tissues, stimulation of lipogenesis, glycogenogenesis, a decrease in the rate of glucose production by the liver, etc. For doses of 0.2–0.4 IU/kg 50%, the maximum effect occurs in the range from 3– 4 hours to 14 hours after administration. After subcutaneous administration, a pharmacodynamic response proportional to the administered dose (maximum effect, duration of action, overall effect) was observed. After subcutaneous injection, detemir binds to albumin through its fatty acid chain. Thus, in a state of sustained action, the concentration of free unbound insulin is significantly reduced, which leads to a stable level of glycemia. The duration of action of detemir at a dose of 0.4 U/kg is about 20 hours, so the drug is prescribed twice a day for most patients. In long-term studies (6 months), fasting plasma glucose levels in patients with type 1 diabetes mellitus were better compared with isophane insulin prescribed in basal/bolus therapy. Glycemic control (glycosylated hemoglobin - HbA1c) during treatment with insulin detemir was comparable to that during treatment with isophane insulin, with a lower risk of nocturnal hypoglycemia and no weight gain during its use. The nocturnal glucose control profile is flatter and more even with insulin detemir compared to isophane insulin, which is reflected in a lower risk of developing nocturnal hypoglycemia. The maximum concentration of insulin detemir in the blood serum is achieved 6–8 hours after administration. With a twice daily administration regimen, steady-state concentrations of the drug in the blood serum are achieved after 2–3 administrations. Inactivation is similar to that of human insulin preparations; all metabolites formed are inactive. Results from in vitro and in vivo indicate that there are no clinically significant interactions between insulin detemir and fatty acids or other drugs that bind to blood proteins. The half-life after subcutaneous injection is determined by the degree of absorption from the subcutaneous tissue and is 5–7 hours, depending on the dose. With subcutaneous administration, serum concentrations were proportional to the administered dose (maximum concentration, degree of absorption). Special Populations Pharmacokinetic properties were studied in children (6–12 years) and adolescents (13–17 years) and compared with adults with type I diabetes mellitus. No differences in pharmacokinetic properties were identified. There were no clinically significant differences in the pharmacokinetics of insulin detemir between elderly and young patients, or between patients with impaired renal and hepatic function and healthy patients.
Introduction
Currently, a full arsenal of medications is available to achieve compensation for patients with diabetes mellitus (DM).
However, despite the large number of groups of drugs for the treatment of diabetes, insulin remains the most effective (“golden”). His discovery is one of the most significant events in medicine and pharmacology, as evidenced by three Nobel Prizes awarded for his work with the insulin molecule. Very soon the medical community will celebrate the 100th anniversary of the creation of this unique drug. The history of insulin is the history of medical advances. The discovery of insulin determined a radically new approach to the treatment of diabetes. Only 2 years passed from the discovery of the drug to the start of its industrial production and clinical use. The first injection of insulin into a human was made on January 11, 1922, to a 14-year-old teenager with type 1 diabetes (T1DM). From that moment on, the grand era of insulin therapy began. The following significant milestones in the development of insulin therapy can be identified: the isolation of insulin in 1921, the use of animal (bovine) insulin in 1922 (Fig. 1), the creation of protamine-zinc insulin in 1936, neutral protamine Hagedorn in 1946. and Lente insulin in 1952, the use of recombinant technologies for the synthesis of genetically engineered human insulins in 1977 [1–3]. The advent of recombinant DNA technology in the 1980s. made it possible to change the molecular structure of insulin and modify those areas of ligand-receptor interaction that remained unchanged when using “human insulins” [4]. The first generation of basal insulins was quite far from the physiological profile of insulin. The pharmacokinetic profile of NPH insulin (neutral protamine Hagedorn) reached a maximum 6–10 hours after injection, followed by a steady decrease [5]. The rate of absorption of NPH insulin constantly changes over time and creates poorly predictable pharmacokinetic profiles, increasing the risk of developing hypoglycemic episodes, especially nocturnal hypoglycemia [6]. A new era in insulin therapy was the development in the 1990s. rapid-acting insulin analogues and basal analogues in the early 2000s (Fig. 2). The first basal analogue was insulin glargine, created in 2000, followed by insulin detemir in 2005. Despite significant progress made in understanding the pharmacokinetics, pharmacodynamics and mechanisms of action of insulin, issues related to the lack of complete imitation of the endogenous insulin profile still remain relevant. Long-acting insulins are the most used insulin preparations in clinical practice, which is associated with the mass of patients with type 2 diabetes (T2DM) in the population and the need to prescribe them insulin therapy several years after diagnosis. It is basal insulins that become the starting insulin when prescribing insulin to patients with T2DM [7].
The main goals of diabetes therapy include achieving and maintaining glycemic targets necessary to prevent late complications of diabetes and a low risk of hypoglycemia [8]. The results of population-based studies indicate that hypoglycemia is the most significant factor preventing the achievement of adequate glycemic control and significantly worsens the quality of life of patients [9]. Traditional preparations of human insulin have variability in absorption and significant differences in pharmacodynamic effect, which determine the failure to achieve stable and long-term plasma insulin levels. The variability of insulin action causes significant fluctuations in glycemia and the likelihood of developing hypoglycemic conditions.
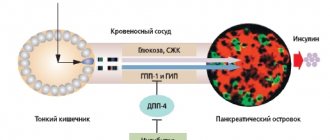
In order to solve these problems, a long-acting insulin analogue has been created, which has a number of distinctive properties and has a prolonged effect - insulin detemir. Insulin detemir (Levemir®) is a neutral, soluble, long-acting basal analogue of human insulin with a flat action profile, which is produced by recombinant DNA biotechnology using a strain of Saccharomyces cerevisiae. Insulin detemir provides a replication close to the basal physiological insulin profile, which provides the advantage of a more predictable action profile compared to NPH insulin [10]. The molecular structure of insulin detemir differs from the structure of human insulin in the absence of threonine at position B30 and the addition of myristic acid to lysine at position B29 (a fatty acid residue of 14 carbon atoms - C14; Fig. 3). This structure of the molecule made it possible to obtain an insulin analogue, which remains in a dissolved state after the injection. Insulin detemir molecules in the finished solution are combined into hexamers. When insulin detemir enters the interstitial fluid of subcutaneous fat, self-association of hexamers occurs with the formation of dihexamers due to contact between the fatty acid chains of insulin molecules [10], as well as the binding of hexamers to albumin. With further dilution, the hexamers decompose into dimers and monomers, which can slowly pass through the capillary wall. Most monomers (about 98%) quickly bind to serum albumin molecules. Free monomers of insulin detemir leave the circulation and reach target tissues, in which insulin detemir binds to the insulin receptor on the membrane of target cells, which triggers the signaling process as the main biological effect - signal transmission to the cells of target organs [11, 12].
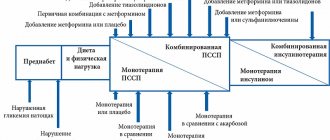
Insulin detemir is widely used in T1DM and T2DM in combination with short-acting/ultra-short-acting insulins, as well as with representatives of almost all other groups of non-insulin antidiabetic drugs: detemir + metformin, detemir + sulfonylureas/glinides, detemir + DPP-4, detemir + inhibitors α-glucosidases, detemir+aGLP-1, detemir+iNGLT-2.
Over a period of more than 15 years since the start of using insulin Detemir, a lot of observations and significant clinical experience in the use of this basal insulin analogue have been accumulated.
Clinical efficacy and safety of insulin detemir
The presented data from meta-analyses and randomized clinical trials showed that insulin detemir has high clinical efficacy in all age groups. There is a significant reduction in the risk of all hypoglycemic conditions in patients, incl. night, regardless of the type of diabetes and its duration. The results of all clinical studies conducted confirm the greater predictability of the action of insulin detemir compared to NPH insulin. The international observational study PREDICTIVE™ 303 (n=5603) assessed the effectiveness of insulin detemir therapy in patients with T2DM using a simplified self-titration algorithm (A1) compared with a standard physician titration algorithm (A2) in outpatient primary care centers over 6 months [14]. The study showed that in patients switched from insulin glargine, glycosylated hemoglobin (HbA1c) levels decreased after 26 weeks from 8.4 to 8.0% in the A1 titration group and from 8.4 to 8.2% in the A2 titration group (p =0.052).
In patients switched from NPH insulin therapy to insulin detemir, the HbA1c level decreased from 8.2 to 8.0% in group A1, from 8.3 to 8.2% in group A2 (p = 0.38). The average daily doses of insulin detemir after 26 weeks of therapy were 0.69 and 0.57 U/kg in titration groups A1 and A2, respectively, in patients transferred from therapy with insulin glargine 100. In the group of patients who initially received NPH insulin, doses were 0. 74 and 0.53 U/kg in groups A1 and A2, respectively. An assessment of the frequency of hypoglycemia (episode/patient/month) showed a decrease after 26 weeks from 0.72 initially to 0.56 in titration group A1, from 0.74 to 0.39 in group A2 in patients switched to insulin detemir therapy from insulin glargine. In the group of previous NPH insulin therapy, a similar reduction in the incidence of hypoglycemia was shown. A study in patients switched from basal insulin glargine±PSSP (oral antihyperglycemic agents) or NPH insulin to insulin detemir in outpatient primary care centers showed significant improvement in glycemic control along with moderate weight loss and a significant reduction in the risk of hypoglycemia [14 ].
An assessment of the effectiveness and safety of insulin detemir therapy in everyday clinical practice (PREDICTIVE™ study) of 2377 patients of the European cohort with T2DM, who had not previously received insulin, with poor glycemic control on oral glucose-lowering therapy showed that 3 months after the start of insulin detemir therapy, a significant decreased HbA1c levels and low incidence of hypoglycemia without severe episodes. Greater weight loss was observed in patients who discontinued prior sulfonylurea or thiazolidinedione therapy compared with those who continued oral therapy unchanged [15]. In patients with T2DM, treatment with insulin detemir in combination with previous PSSP therapy or as a result of replacing previous basal insulin provided rapid and significant improvement in glycemic control, reduced risk of hypoglycemia and weight loss [16].
Similar results were obtained in the PREDICTIVE™ study in 682 patients with T1DM in the European cohort (38% men, mean age 40.3 years, diabetes duration 19.7 years, body mass index [BMI] 25.3±4.1 kg /m2) who received insulin detemir therapy as a basal component of a basal-bolus (BB) therapy regimen for 6 months. Previous therapy was insulin glargine in the BB therapy regimen. Over 29 weeks of follow-up, the overall incidence of hypoglycemic episodes decreased from 67.3 patient-years to 47.3 (p < 0.0001). A reduction in the incidence of severe and nocturnal episodes of hypoglycemia was shown (up to 2.0 and 9.0 patient-years; p < 0.0001 for both comparisons). The average HbA1c level and intra-individual variability of fasting glycemia also decreased significantly. Patients showed a significant decrease in average body weight, most pronounced in patients with high BMI (29–31 kg/m2 [-1.7 kg], >31 kg/m2 [-2.0 kg]). Transferring patients treated with insulin glargine in a BB regimen to insulin detemir therapy in a BB regimen provides improved glycemic control and a reduction in the frequency of hypoglycemia with a moderate decrease in body weight [17]. Findings from the PREDICTIVE™ study indicate that additional benefits in terms of efficacy and safety of therapy can be obtained by replacing basal insulin glargine with insulin detemir in beta-blocker regimens for patients with type 1 diabetes.
Unidirectional changes in carbohydrate metabolism have also been reported in several studies of patients with T1DM. In an open-label, parallel-group study (16 weeks), 408 patients with T1DM were randomized to either insulin detemir or NPH insulin [18]. Patients received the ultra-short-acting insulin analogue aspart as a bolus insulin. HbA1c and fasting plasma glucose (FPG) levels over time in the insulin detemir treatment group were significantly lower compared to NPH insulin (p=0.027). The variability of plasma glucose values before breakfast, according to self-monitoring of glycemia, was lower in insulin detemir therapy, as well as mild episodes of hypoglycemia (25%; p = 0.046; 32%; p = 0.002, respectively), compared with the group of patients on NPH insulin therapy. Thus, overall glycemic control during therapy with insulin detemir was significantly better compared with therapy with NPH insulin [18].
In a 6-month, comparative, multinational, parallel-group study, 448 patients with T1DM were randomized to treatment with insulin detemir or NPH plus rapid-acting insulin aspart [19]. While rates of glycemic control were similar between groups, intra-individual variability was significantly lower with insulin detemir therapy compared with NPH insulin therapy (p<0.001). In addition, therapy with insulin detemir was accompanied by a significant reduction in the risk of all hypoglycemia, incl. night (by 22 and 34%, respectively). At the end of the study, body weight was significantly lower in patients in the insulin detemir treatment group.
Thus, the results of a number of studies on the treatment of patients with T1DM and T2DM suggest that additional benefits in terms of the effectiveness and safety of therapy can be obtained by replacing the basal insulins NPH and glargine with insulin detemir. Insulin detemir showed its greatest effectiveness and safety when administered 2 times a day at a dose of 0.4 U/kg body weight [20, 21].
Duration of action of insulin detemir and its variability
An important disadvantage of traditional human insulin preparations is their unpredictable activity, which is associated with inconsistent absorption of the drugs. The period of absorption of 50% insulin detemir from the subcutaneous fat depot is 10.2 ± 1.2 hours compared to 2.0 ± 0.1 for monomeric acylated insulin [22]. The prolonged effect of Levemir® is due to the pronounced self-association of insulin molecules at the injection site and the binding of drug molecules to albumin through a connection with the side fatty acid chain; 98–99% of circulating insulin detemir is bound to albumin, which allows for slower transepithelial transport from the blood to the interstitial tissue compared to human insulin, i.e. further prolongation of the action of detemir is associated with circulation in the bloodstream, where its concentration is maintained due to a strong connection with serum albumin. These mechanisms of delayed distribution provide a more predictable and reproducible profile of absorption and action of Levemir® compared to isophane insulin [10, 23].
To assess the hypoglycemic effects of insulin detemir, groups of healthy volunteers and patients with T1DM and T2DM were examined using the euglycemic clamp method [24, 25]. The average duration of action of insulin detemir was about 24 hours for patients with T1DM and more than 24 hours for patients with T2DM [22], but it varied among individual patients. Insulin detemir is characterized by a dose-dependent duration of action, which was demonstrated in a randomized, double-blind, crossover study of patients with T1DM [26, 27]. Using a 24-hour euglycemic clamp, patient parameters were monitored throughout the day in response to the administration of NPH insulin (0.3 U/kg) and insulin detemir (0.1 U/kg; 0.2; 0.4; 0.8; 1.6 units/kg). The study showed that when administered insulin detemir, a flat, prolonged pharmacodynamic profile is formed with high predictability of action compared to NPH insulin.
In the present study, a linear relationship between the dose of insulin detemir and the response in the form of the duration of action was determined: the duration of action of insulin detemir at a dose of 0.4 U/kg was 20 hours; when the dose was increased to more than 0.4 U/kg, the duration of action increased and reached 22–24 hours.
Currently, assessment of the effectiveness of diabetes treatment is not limited to the level of HbA1c, but is determined by the range and frequency of fluctuations in daily glycemia. In this regard, another significant problem of insulin therapy is significant insulin variability, leading to fluctuations in blood glucose levels and an increase in the frequency of hypoglycemia, which in turn reduces the likelihood of achieving glycemic targets [27]. At the end of the 20th century. About 30 different criteria have been proposed to characterize glycemic variability, to obtain which the results of glycemic self-monitoring and continuous daily monitoring are used.
The lower variability of the action of insulin detemir compared to other basal insulins (NPH and glargine 100) is associated with a number of its properties. The absorption of insulin detemir is not affected by resuspension or dissolution of microprecipitates. Detemir is a soluble insulin, which means that the drug does not need to be resuspended, and precipitates do not form at the injection site [4, 5, 10]. Reversible binding to plasma albumin determines the buffering effect of detemir, in which there is no immediate change in the pharmacodynamic response. In this regard, detemir has a flatter, longer and more predictable action profile than NPH insulin, which helps reduce the risk of diabetes complications [25]. The action profile of insulin detemir is significantly less variable compared to insulin isophane and insulin glargine (AUCGIR, 0–24 hours for insulin detemir is 0.074, 0.466 for insulin isophane and 0.231 for insulin glargine; GIRmax for insulin detemir is 0.053, 0.209 – for insulin isophane and 0.130 – for insulin glargine) [10].
In a randomized, double-blind study, 54 patients with T1DM were administered a single dose of 0.4 U/kg insulin detemir, glargine 100, or NPH insulin under euglycemic clamp conditions (target blood glucose concentration 5.5 mmol/L) [28]. The results of the study showed that insulin detemir is associated with less intra-individual variability in glucose utilization than NPH insulin and insulin glargine (Fig. 4).
A single-center, parallel-group study (MTB Medizintechnik, Ulm, Germany) aimed to evaluate the variability in pharmacodynamics and pharmacokinetics for the insulins detemir, NPH, and glargine in 54 patients with T1DM (age 38 ± 10 years, duration of diabetes 18 ± 9 years). It was shown that when comparing the variability of insulin detemir with two basal insulins (NPH insulin and insulin glargine), detemir had a more predictable glucose-lowering profile with significantly less variability compared to other insulins [27, 29]. The lower variability of insulin detemir is demonstrated by the 95% range of predicted incidence of hyper- and hypoglycemia during therapy with insulin detemir compared to NPH and glargine insulins. Risk of severe hypoglycemia (50% lower than usual average glycemia): approximately 2 times per year with insulin detemir, 57 times per year with NPH insulin and 27 times per year with insulin glargine [29]. These results indicated that the lower variability of insulin detemir serves as a significant factor for patients on insulin therapy, predicting improved metabolic control with fewer fluctuations in blood glucose levels with day-to-day therapy.
Hypoglycemia
One of the most important consequences of insulin variability is the increased risk of hypoglycemia, which serves as a major barrier to effective and safe diabetes management. To evaluate the benefits of insulin detemir in reducing the risk of hypoglycemia while achieving diabetes compensation, a meta-analysis of four open-label, randomized, multicenter clinical trials of 1180 patients with type 1 diabetes was conducted. The frequency of severe and mild hypoglycemia was assessed during therapy with insulin Detemir administered 1 or 2 times a day (number of episodes per 1 patient per month) and during therapy with NPH insulin (n=810). The assessment showed that the risk of hypoglycemia was 22% lower in the insulin detemir group compared with the NPH insulin group (p < 0.001). Moreover, as HbA1c levels improved, the risk of hypoglycemia further tended to decrease [30]. According to a Cochrane review (2009), the risk of symptomatic and nocturnal and severe episodes of hypoglycemia was on average 16 and 18%, 34 and 37%, 30 and 50% lower with insulin glargine and detemir than with NPH insulin, respectively [8 ].
In a 26-week multicenter randomized trial involving 322 patients with T1DM, insulin detemir demonstrated a 32% and 72% reduction in the risk of nocturnal hypoglycemia and severe hypoglycemia, respectively, compared with insulin glargine [31], which demonstrated less variability in the effect of insulin detemir compared with other basal insulins [32].
Efficacy and safety of insulin detemir in different patient groups
Proven benefits of using insulin detemir in the treatment of diabetes in pregnant patients
Diabetes mellitus during pregnancy leads to a host of fetal and maternal complications. Insulin is the most effective pharmacologic agent for controlling hyperglycemia during pregnancy and may limit adverse outcomes. Since 2012, insulin detemir has been approved for use in the treatment of pregnant women with diabetes.
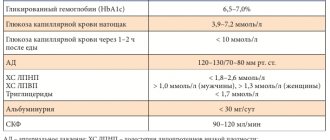
The effectiveness and safety of insulin detemir in pregnant women with type 1 diabetes was assessed in comparison with NPH insulin [33]. The proportion of patients achieving target HbA1c levels (6.0%) at 24 and 36 weeks of pregnancy did not differ significantly between detemir and NPH insulin therapy. While FPG was significantly lower in the group of pregnant women receiving insulin detemir therapy compared to NPH insulin (see table). Body weight gain in patients receiving insulins detemir and NPH was identical and amounted to 11.5 and 11.0 kg, respectively.
The study found no difference between pregnancy outcomes in patients treated with insulin detemir and NPH: birth of a child weighing less than the 10th percentile or above the 90th percentile for gestational age and sex, preterm birth (<37 gestational weeks), early fetal death (<22 gestational weeks), perinatal mortality, presence of congenital malformations [34]. The body weight of newborns was 3504 and 3571 g, respectively, in the detemir and NPH insulin groups. In the group of women receiving insulin detemir therapy, 46% of infants were large for gestational age, 19% had macrosomia, and in the NPH insulin group, 54% of infants were large for gestational age and 26% had macrosomia.
Perinatal outcomes serve as a clear reflection of the course of pregnancy. A randomized controlled trial aimed to compare the effectiveness and safety of insulin detemir and NPH insulin in pregnant women with T1DM. Two groups of patients were randomized to insulin detemir (n=152) and NPH insulin (n=158) either in the 12 months before pregnancy or at 8–12 weeks of pregnancy. Results of the study: 128 and 136 newborn children in groups with detemir and NPH-insulin therapy, 11 and 9 – early fetal losses, 2 and 1 – perinatal death, respectively [35]. As a result of pregnancy, 16 children had developmental defects; detemir – 8 (5.6%), NPH – 8 (5.5%). The incidence of side effects was similar between groups. Therapy with insulin detemir did not cause safety problems for the mother and fetus; the drug was tolerated as well as NPH insulin [36, 37].
Insulin detemir and T1DM in children and adolescents: issues of effectiveness and safety
A special group of patients are children and adolescents with diabetes.
A randomized, controlled, 6-week parallel group study comparatively assessed the effectiveness and safety of insulins detemir and NPH in combination with insulin aspart for type 1 diabetes in children and adolescents. 347 children aged 6–17 years were included in the study [38]. Both groups achieved similar HbA1c levels with a more predictable glycemic profile of insulin detemir and less intra-individual glycemic variability. There was a significantly lower risk (26%) of nocturnal hypoglycemic episodes on insulin detemir therapy compared with NPH insulin, and a significantly lower BMI in children and adolescents receiving insulin detemir therapy. The study showed that the combination of two insulin analogues in the context of BB therapy (detemir + aspart) makes it possible to realize the advantages of each of these drugs and obtain a more pronounced clinical effect: an effective decrease in HbA1c levels, less variability of glycemia, a significant decrease in hypoglycemic states, especially at night, and lower BMI values [39].
Efficacy and safety of BB therapy with insulin detemir and NPH in combination with insulin aspart in children 2–16 years old with type 1 diabetes [40]
Insulin detemir showed similar HbA1c levels to NPH insulin at 12, 26, 38 and 52 weeks in the overall patient cohort, proving its effectiveness. At the same time, significantly lower variability was determined at all observation points compared to NPH insulin in the general cohort of children and adolescents (SD 3.01 versus 3.68 mmol/l; p<0.001). When assessing the dynamics of body weight (SDS, Z-score) in children, significant differences were obtained: the SD-score of body weight was 0.18 on insulin detemir therapy, while on NPH insulin therapy it was 0.33 (average difference - -0 .15, 95% CI – -0.23–-0.07; p<0.001).
The most significant indicator in the study was the assessment of hypoglycemic episodes over 52 weeks of therapy. There were 3 episodes of hypoglycemia in the absence of nocturnal hypoglycemia in the insulin detemir group compared to 15 episodes in the NPH insulin group, incl. 6 nocturnal hypoglycemia.
In other studies in groups of children aged 6–17 years with T1DM, HbA1c at 26 weeks of follow-up was not significantly different between insulin detemir and NPH insulin recipients [41], with a significant difference in self-measurement FPG in patients on insulin detemir compared with with NPH insulin (p<0.001). FPG levels were significantly lower in children aged 6–17 years treated with insulin detemir compared with NPH insulin. The mean BMI Z-score was also significantly lower in the insulin detemir group than in patients on NPH insulin (0.08 and 0.26, respectively; p < 0.001) [42].
In another study, the change in the mean standard deviation (SD) of body weight of patients aged 2–16 years was -0.12 versus 0.04 (p < 0.001) in the detimir and NPH insulin groups, respectively [43]. The average basal insulin dose in the insulin detemir group was 32.2 and 31.0 U/day in the NPH insulin group in children aged 6–17 years [44]. According to the results of another study, in recipients of insulin detemir and NPH-inulin, the basal dose was 0.60 and 0.58 U/kg body weight/day in the age group 2–16 years [45].
Thus, insulin detemir has demonstrated its effectiveness and safety in the pediatric age group and is indicated for the treatment of diabetes in adolescents and children aged 1 year and older.
The role and place of insulin detemir in the treatment of patients of older age groups
One of the most important principles of diabetes therapy is balancing glycemic control with the risk of side effects. Elderly patients are of particular concern given a range of clinical, laboratory and psychosocial features. This is primarily the presence of combined multiple organ pathology, incl. pathologies of the liver and kidneys, cardiovascular risks, nonspecific complaints and asymptomatic course, micro- and macroangiopathies even when diabetes is detected, impaired cognitive functions. In this regard, assessing the effectiveness and safety of the drug is of great importance for elderly people.
The international open-label, non-interventional study A1chieve assessed the clinical safety and effectiveness of insulin detemir in the age group 65 years and older with T2DM when switching to insulin detemir with PSSP or NPH insulin. The 24-week study subanalysis included 15,241 patients. Treatment with insulin detemir during this period resulted in significant improvements in glycemic control and quality of life as a result of a reduction in the incidence of severe and nocturnal hypoglycemia compared with the baseline incidence (p < 0.05) [46]. The data obtained on the dynamics of body weight were interesting: 24 weeks of therapy led to significant weight loss in patients switched to insulin detemir from another insulin, while those patients who first received insulin therapy had a slight increase in body weight [46 ].
Elderly patients are especially vulnerable to hypoglycemia. An observational study conducted over 24 weeks in real-world clinical practice evaluated insulin detemir therapy, involving 3219 investigators and 2817 project sites from ten countries [47, 48]. Insulin detemir was administered as a single dose to patients with T2DM in groups older and younger than 75 years of age who were treated with one or more oral antidiabetic drugs. The total cohort consisted of 17,374 participants, of whom 2,398 (14%) were over 75 years of age. The initial HbA1c level was similar in the groups of patients older and younger than 75 years (HbA1c – 8.8±1.5 and 8.9±1.6%, respectively). Evaluation of the effectiveness of insulin detemir therapy showed that after 24 weeks of treatment, a similar decrease in HbA1c levels was also observed in the two groups: 7.6 ± 1.1 and 7.5 ± 1.2%. The incidence of severe hypoglycemia (patient-year) decreased in the study in both age groups: from 0.057 to 0.007 in patients over 75 years of age and from 0.042 to 0.005 in patients under 75 years of age. Body weight assessment showed a similar reduction in mean values in both groups: -0.5 kg in patients over 75 years of age and -0.6 kg in patients under 75 years of age. The result of the study in real clinical practice was evidence of the effectiveness and safety of insulin detemir therapy in the older age group without any association with an increased risk of severe hypoglycemia or weight gain [49].
Conclusion
Experience from real clinical practice shows that patients with diabetes often do not achieve disease compensation. This is largely due to significant changes in glycemia during the day and a high level of glycemic variability. The long-acting insulin analog insulin detemir is an effective and well-tolerated basal insulin therapy for patients with T1DM and T2DM. The use of insulin detemir as part of BB therapy or as basal therapy together with PSSP helps to improve glycemic control, lower glycemic variability, and reduce the risk of hypoglycemic conditions, incl. nocturnal hypoglycemia, less weight gain compared to NPH insulin. Thus, modern insulin therapy regimens using insulin analogues, incl. insulin detemir, allow you to achieve compensation for carbohydrate metabolism, prevent the formation and progression of complications, helping to improve the quality of life of patients with diabetes.
Use of the drug Insulin detemir
Intended for subcutaneous administration. The dose is determined individually in each specific case. Insulin detemir should be prescribed 1 or 2 times a day based on the patient's needs. Patients requiring twice daily dosing for optimal blood glucose control may administer the evening dose either with the evening meal, at bedtime, or 12 hours after the morning dose. Insulin detemir is injected subcutaneously into the thigh, anterior abdominal wall or shoulder. injection sites should be changed even when injecting into the same area. As with other insulins, in elderly patients and patients with renal or hepatic impairment, blood glucose levels should be monitored more carefully and the dose of insulin detemir should be adjusted individually. Dose adjustment may also be necessary if the patient increases physical activity, changes his usual diet, or has a concomitant illness.
results
As a result of an indirect comparison of the clinical effectiveness of insulin glargine 300 U/ml and insulin detemir, carried out through a common comparator - insulin glargine 100 U/ml - and based on the calculation of the OR based on the ratio of the number of patients who achieved/did not achieve the target HbA1c level (see table 1), the following results were obtained (Table 6).
Table 6
Results of an indirect comparison of the clinical efficacy and tolerability of insulin glargine 300 U/ml with insulin detemir
| Comparison direction | Achieving target HbA1c level | Heavy hypoglycemia | |||
| OSH | 95% CI | OSH | 95% CI | ||
| Insulin glargine 300 units/ml → insulin glargine 100 units/ml | 1,20 | (1,02; 1,42) | 0,58 | (0,40 | 0,82) |
| Insulin glargine 100 units/ml → insulin detemir | 1,06 | (0,92; 1,22) | 1,01 | (0,86 | 1,12) |
| Indirect comparison insulin glargine 300 U/ml → insulin detemir | 1,27 | (1,02; 1,58) | 0,72 | (0,56 | 0,88) |
The probability of achieving the target HbA1c level <7% when using insulin glargine 300 U/ml is significantly higher (20%) than when using insulin glargine 100 U/ml. The OR for comparison insulin glargine 100 U/ml → insulin detemir was 1.06, with the confidence interval crossing the value of 1.0, which indicates comparable effectiveness of these insulin analogues and coincides with data from direct comparative studies (in particular, when analyzing the practice of insulin therapy in 16,341 patients with type 2 diabetes [32]). An indirect comparison of the calculated OR values for insulin glargine 300 U/ml and insulin detemir showed that the probability of patients achieving an HbA1c level <7% when using glargine was significantly higher (by 27%). The calculated rate of achieving compensation for type 2 diabetes over a 6-month period was 25.3% for insulin glargine 300 U/ml and, taking into account the above OR, 19.9% for insulin detemir. Direct costs for drugs are presented in table. 1, they are calculated on the basis of dosages determined in the input of indirect comparison of drugs based on the patient’s body weight of 70 kg. It can be seen that the daily dosage of insulin detemir, based on the results of indirect comparison, is expected to be higher by 13.8% in comparison with insulin glargine 300 U/ml. At the same time, taking into account the difference in the cost of these drugs, the annual course of insulin glargine 300 U/ml does not differ in cost from that of insulin detemir. These costs, as we will show below, are not always the economic basis for making a decision to prescribe a particular insulin, since they do not reflect the total costs (direct and indirect) of treatment.
The OR parameter in relation to the probability of developing a severe hypoglycemic event is statistically significantly lower for insulin glargine 300 U/ml (see Table 6). If we assume that in the insulin glargine 300 U/ml group one severe hypoglycemia occurs during the year, then 1.38 such events are expected in the insulin detemir group. At the same time, the calculation shows a difference of more than 5,000 rubles. for the treatment of severe hypoglycemia in groups (Table 8).
In table 7 presents direct medical and non-medical (payment of sick leave), as well as indirect (loss of GDP) costs (expenses). Calculations indicate that, in general, the costs of treatment with insulin glargine 300 U/ml do not exceed those for treatment with insulin detemir. At the same time, from a medical point of view, insulin glargine has some advantages compared to insulin detemir:
- all 100% of patients can use insulin glargine 300 U/ml once a day, while the percentage of those receiving detemir once a day for type 2 diabetes is no more than half [33], which is not entirely convenient for long-term treatment and may based on the fact that in 52.7% of cases after 24 months. patients refuse to administer the drug [32], and this can have an extremely negative impact on the prognosis of the disease;
- the number of symptomatic and severe hypoglycemia is less;
- “flexible” dosing of insulin glargine 300 U/ml (3 hours before/after the usual administration time) is possible without compromising the clinical effect [34].
Table 7
Costs depending on achievement / failure to achieve compensation for diabetes 2 (1 case, rub.)
| Cardiovascular event | Direct medical weighted average costs when HbA1c <7% is not achieved (RUB) | Direct medical weighted average costs when HbA1c <7% (RUB) | Direct non-medical costs (RUB) | Weighted average direct non-medical costs when HbA1c <7% is not achieved (RUB) | Weighted average direct non-medical costs when HbA1c <7% (RUB) | Indirect costs (RUB) | Weighted average indirect costs when HbA1c <7% is not achieved (RUB) | Weighted average indirect costs when HbA1c <7% (RUB) |
| Arterial hypertension | 17 780,87 | 12 624,42 | 6 327,22 | 3 028,87 | 2 150,50 | 15 463,43 | 7 402,43 | 5 255,72 |
| Heart rhythm disturbances | 11 065,04 | 7 856,18 | 6 327,22 | 1 288,70 | 914,97 | 15 463,43 | 3 149,51 | 2 236,15 |
| Angina pectoris | 11 013,27 | 7 819,42 | 11 863,54 | 2 243,71 | 1 593,04 | 28 993,92 | 5 483,53 | 3 893,30 |
| Chronic heart failure | 4 733,32 | 3 360,66 | 11 863,54 | 1 339,65 | 951,15 | 28 993,92 | 3 274,05 | 2 324,57 |
| Myocardial infarction | 12 242,48 | 8 692,16 | 96 490,09 | 6 818,25 | 4 840,96 | 235 817,24 | 16 663,49 | 11 831,08 |
| Stroke | 11 234,34 | 7 639,35 | 120 217,16 | 5 829,82 | 3 964,27 | 293 805,09 | 14 247,80 | 9 688,50 |
| Total | 68 069,32 | 47 992,19 | 20 549,01 | 14 414,89 | 50 220,81 | 35 229,32 |
Table 8
Costs of treating type 2 diabetes with insulin glargine 300 U/ml and insulin detemir (per 1 patient/year, rub.)
| Cost type | Insulin glargine 300 units/ml | Insulin-detemir | Difference insulin glargine 300 units/ml - insulin detemir |
| Direct costs | |||
| Costs of insulin therapy | 15 856,33 | 15 682,59 | 173,74 |
| Costs for treatment of cardiovascular complications* | 63 882,66 | 64 064,08 | -181,42 |
| Costs of treating severe hypoglycemia** | 15 180,00 | 20 948,84 | -5 768,84 |
| Payment for temporary disability | 19 894,88 | 19 325,28 | -569,60 |
| Total direct costs | 114 813,87 | 120 020,79 | -6 346,12 (-4,3 %) |
| Indirect costs | |||
| Lost GDP*** | 46 226,00 | 47 230,12 | -1 004,12 (-2,1 %) |
| Total total costs | 161 039,87 | 167 250,91 | -7 350,24 (-3,7 %) |
Notes: * - taking into account all events for patients who both achieved and did not achieve the target HbA1c level; ** - scientifically based assumption about the number of severe conditions (see text); *** - as a result of temporary disability.
It is appropriate to note that for those for whom insulin detemir is indicated, it should be prescribed, despite the above arguments in favor of insulin glargine 300 U/ml.
A two-way sensitivity analysis for changes in prices for the drugs being compared (Table 9) confirms the conclusions of the main scenario about the economic feasibility of using insulin glargine 300 U/ml in terms of the total costs of treating type 2 diabetes during the year (Table 8). One may, of course, consider that a potential cost reduction of 4-5% is not so significant, but if we remember the number of patients who need effective and safe insulin therapy, the economic benefits of using cheaper therapy programs become obvious.
Table 9
Sensitivity analysis regarding changes in prices for compared drugs
| Change in price for insulin detemir (per unit) | Change in price for insulin glargine 300 units/ml (per unit) | ||||||||||
| -50 % | -40 % | -30 % | -20 % | -10 % | 0 % | 10 % | 20 % | 30 % | 40 % | 50 % | |
| 30 % | -18,5 % | -7,5 % | -16,5 % | -15,4 % | -14,4 % | -13,3 % | -2,3 % | -1,3 % | -0,2 % | 0,8 % | 1,9 % |
| 20 % | -17,7 % | -16,6 % | -15,6 % | -14,5 % | -13,5 % | -12,4 % | -3,4 % | -0,3 % | 0,7 % | 1,8 % | 2,8 % |
| 10 % | -16,8 % | -15,7 % | -14,7 % | -13,6 % | -12,5 % | -10,5 % | -4,4 % | 0,6 % | 1,7 % | 2,7 % | 3,8 % |
| 0 % | -15,9 % | -14,8 % | -11,5 % | -7,9 % | -5,7 % | -3,7 % | -1,6 % | 1,8 % | 2,7 % | 4,2 % | 4,8 % |
| -10 % | -5,0 % | -3,9 % | -2,8 % | -1,7 % | -0,6 % | 0,4 % | 1,5 % | 2,6 % | 3,7 % | 4,8 % | 5,8 % |
| -20 % | -4,0 % | -2,9 % | -1,8 % | -0,7 % | 0,3 % | 1,4 % | 2,5 % | 3,6 % | 4,7 % | 5,8 % | 6,9 % |
| -30 % | -3,1 % | -2,0 % | -0,9 % | 0,2 % | 1,3 % | 2,4 % | 3,5 % | 4,6 % | 5,7 % | 6,8 % | 7,9 % |
Side effects of the drug Insulin detemir
Adverse reactions observed in patients using insulin detemir are mainly dose-dependent and develop due to the pharmacological effect of insulin. Hypoglycemia is generally the most common side effect. Hypoglycemia develops if the dose of the drug administered is too high relative to the body's need for insulin. Injection site reactions may occur during treatment in approximately 2% of patients. The proportion of patients receiving treatment who are expected to develop side effects is estimated to be 12%. The incidence of side effects during clinical trials is presented below. Metabolic and nutritional disorders: common (1/100, ≤1/10). Hypoglycemia: Symptoms of hypoglycemia usually develop suddenly. They include “cold sweat,” pale skin, increased fatigue, nervousness or tremors, anxiety, unusual tiredness or weakness, disorientation, decreased concentration, drowsiness, extreme hunger, blurred vision, headache, nausea, and palpitations. Severe hypoglycemia can lead to loss of consciousness and/or seizures, temporary or irreversible impairment of brain function, and even death. General disorders and reactions at drug administration sites: common (1/100, ≤1/10). Injection site reactions : Local hypersensitivity reactions (redness, swelling and itching at the injection site) may develop during insulin treatment. These reactions are usually short-lived and disappear with continued treatment. Rare (1/1000, ≤1/100). Lipodystrophy: may develop at the injection site as a result of failure to change injection sites within the same area. Edema: may occur at the initial stage of insulin therapy. These symptoms are usually temporary. Immune system disorders: rare (1/1000, ≤1/100). Allergic reactions: urticaria, skin rash may develop due to hypersensitivity. Signs of hypersensitivity may include itching, sweating, gastrointestinal disorders, angioedema, difficulty breathing, palpitations, and decreased blood pressure. The development of hypersensitivity reactions can be potentially life-threatening. Visual impairment: rare (1/1000, ≤1/100). Refractive errors : Refractive errors may occur during the initial phase of insulin therapy. These symptoms are usually temporary. Diabetic retinopathy . Long-term improvement in glycemic control reduces the risk of progression of diabetic retinopathy. However, intensification of insulin therapy with a sharp improvement in the control of carbohydrate metabolism may lead to a temporary increase in the signs of diabetic retinopathy. Nervous system disorders: very rare (1/10000, ≤1/1000). Peripheral neuropathy : Rapid improvement in glycemic control can lead to a state of acute painful neuropathy, which is usually reversible.
Materials and methods
A direct head-to-head comparison of the use of insulin glargine 300 U/ml with insulin de-temir in real clinical practice has not been carried out. In this regard, an indirect comparison of their clinical effects was carried out through a common reference drug - insulin glargine 100 U/ml, with which comparative studies were carried out with both insulin glargine 300 U/ml and insulin detemir. Since registration clinical trials have a number of limitations due to the peculiarities of their design, studies of the use of the compared drugs in real clinical practice were selected for comparison. The studies selected for analysis are shown in Table. 1.
Table 1
Real-life clinical practice studies used to make indirect comparisons
| № | Description | Total number of patients | Patients who have reached the target HbA1c level/severe hypoglycemia, n | Patients who did not reach the target HbA1c level, n |
| 1 | Retrospective cohort study of the outcomes of insulin glargine 300 U/ml compared with insulin glargine 100 U/ml in adult patients with type 1 and 2 diabetes mellitus based on the Canadian Diabetes Registry [12] | Insulin glargine 100 units/ml 188 | 53/6 | 135 |
| Insulin glargine 300 units/ml 188 | 51/4 | 137 | ||
| 2 | The DELIVER Naive study assessed the achievement of target reductions in HbA1c and the incidence of hypoglycemia in insulin-naive patients receiving insulin glargine 300 U/ml compared with insulin glargine 100 U/ml in real clinical practice [13] | Insulin glargine 100 units/ml 2008 | 432/127 | 1576 |
| Insulin glargine 300 units/ml 1004 | 251/39 | 753 | ||
| Total 1-2 | Insulin glargine 100 units/ml 2196 | 485/133 | 1711 | |
| Insulin glargine 300 units/ml 1192 | 302/43 | 890 | ||
| 3 | Retrospective analysis of data from more than 40 US health care programs collected in the Innovus IMPACT national health care database from 2006 to 2010. The outcomes of using insulin glargine (100 U/ml) and insulin detemir were assessed [14] | Insulin glargine 640 | 153/50 | 487 |
| Insulin detemir 640 | 149/38 | 491 | ||
| 4 | Retrospective analysis of data from real clinical practice in the treatment of patients with type 2 diabetes mellitus who started therapy with insulin glargine (100 U/ml) or insulin detemir based on the General Electric (GE) Centricity EMR database of the USA [15] | Insulin glargine 3467 | 745/198 | 2722 |
| Insulin detemir 915 | 176/47 | 739 | ||
| Total 3-4 | Insulin glargine 4107 | 898/248 | 3209 | |
| Insulin detemir 1555 | 325/85 | 1230 |
Indirect comparison was carried out using the program of the Canadian Agency for Medicines and Health Technologies [16] in accordance with the requirements of the Methodological recommendations for conducting indirect comparisons of medicinal products developed by the Federal State Budgetary Institution "Center for Expertise and Quality Control of Medical Care" of the Ministry of Health of the Russian Federation ("TsEKKMP" of the Ministry of Health RF) [17]. The “surrogate” outcome of achieving target HbA1c values <7% was chosen as the effectiveness criterion for which indirect comparison was carried out. It has been assumed that nocturnal and severe hypoglycemia are classified as severe (plasma glucose level less than 3.0 mmol/l).
The odds ratio (OR) was calculated [18] according to the following schemes:
- OR was determined for comparison: insulin glargine 300 U/ml - insulin glargine 100 U/ml;
- OR was determined for comparison: insulin glargine 100 U/ml - insulin detemir;
- Using a common reference drug—insulin glargine 100 U/ml—the OR was determined for the comparison insulin glargine 300 U/ml → insulin detemir.
OR was calculated using the formula [19]:
OR = (A x D)/(B x C) , where
OR—odds ratio;
A — frequency of achieving HbA1c level <7.0% in the first group;
C — frequency of achieving HbA1c level <7.0% in the second group;
B — frequency of failure to achieve HbA1c level <7.0% in the first group;
D—frequency of HbA1c level <7.0% in the second group.
In this case, a standard four-line table was used (Table 2).
table 2
Four-way table matrix for OR calculations
| Drugs | Achieving HbA1c<7.0% (i, %) | Failure to achieve HbA1c<7.0% (i, %) | Total |
| Drug 1 | A | B | A+B |
| Drug 2 | C | D | C+D |
| Total | A+C | B+D |
The 95% confidence interval (CI) for the calculated OR was determined using the formulas:
for upper bound
for the lower border
When multiple sources were used as inputs in the analysis, the number of patients who did or did not achieve the target HbA1c <7% was summed and the OR was calculated based on the summed values.
The frequency of severe hypoglycemia (plasma glucose less than 3.0 mmol/l) was determined in accordance with data from a retrospective analysis of real clinical practice in 831,456 patients with type 2 diabetes [20]. For insulin glargine 300 U/ml, the probability of severe hypoglycemia requiring hospitalization was 0.07 (frequency / “naive” patient), while for insulin detemir it was statistically significantly higher - 0.15. We calculated the ratio of hypoglycemia based on an indirect comparison of the insulin therapy programs under consideration. There are different approaches to estimating the cost of this iatrogenics; we used data according to which it is estimated at 15,180 rubles. domestic healthcare costs [21].
A scientific assumption has been made that the average daily doses of insulin in real clinical practice correspond to those obtained in a retrospective study [22]. Thus, the calculated average daily dose of insulin glargine 300 units/ml was 0.29 units/kg, and insulin detemir was 0.33 units/kg. Despite the differences in dosages of insulin glargine 100 U/ml and 300 U/ml according to randomized studies, we assumed that their doses per day in real clinical practice do not differ [15][23].
Prices for the compared insulin preparations were determined both according to the state register of maximum selling prices [24] and on the basis of manufacturer data and are presented in Table. 3.
Table 3
Costs of insulins used in the analysis
| INN | Trade name | Package volume (IU) | Manufacturer | Cost of packaging, rub. | Price with 10% VAT and 11.98% TN* | Cost of 1 unit, (rub.) | Daily dose (IU/kg) | Daily dose (ED) | Cost of a year of therapy (rub.) |
| Insulin glargine 300 units/ml | Toujeo SoloStar | 1350 | ZAO Sanofi-Aventis Vostok, Russia | 2 343,29** | 2 886,42 | 2,14 | 0,29 | 20,3 | 15 856,33 |
| 2250 | 3 905,23** | 4 810,38 | 2,14 | ||||||
| Insulin detemir | Levemir FlexPen | 1500 | Novo Nordisk A/C, Denmark | 2 263,68 | 2 788,36 | 1,86 | 0,33 | 23,1 | 15 682,59 |
Notes: * VAT - value added tax; TN—regulated wholesale markup [25]; ** — the cost that the manufacturer plans to re-register in accordance with the Rules [26].
When assessing the burden on the healthcare system budget and the burden of the disease, we took into account data on the frequency of cardiovascular complications (cardiovascular events - CV events) in patients with type 2 diabetes according to the data of the domestic pharmacoepidemiological study FOR-SITE-DM2 [27] (Table 4).
Table 4
Incidence of cardiovascular diseases in type 2 diabetes mellitus [27]
| Cardiovascular disease | Frequency of occurrence, % |
| Arterial hypertension | 69,10 |
| Heart rhythm disturbances | 29,40 |
| Angina pectoris | 27,30 |
| Chronic heart failure | 16,30 |
| Myocardial infarction | 10,20 |
| Stroke | 7,00 |
The cost of treating exacerbations was calculated taking into account the DRG tariffs (Table 5). The frequency of percutaneous coronary intervention (PCI) for myocardial infarction is 75%, and therefore the weighted average cost of treating myocardial infarction will be RUB 109,087.80. The base rate for one completed case of treatment in a 24-hour hospital, according to the SGBP for 2022, is RUB 34,713.70. [28].
Table 5
Cost of treatment of exacerbations of CVD according to DRG tariffs [29]
| Disease | KSG | Cost factor | Cost of a completed case, rub. | Average cost, rub. | |
| Myocardial infarction | st13.001 | Unstable angina, myocardial infarction, pulmonary embolism (level 1) | 1,42 | 49 293,454 | 75 675,87 |
| st13.002 | Unstable angina, myocardial infarction, pulmonary embolism (level 2) | 2,81 | 97 545,497 | ||
| st13.003 | Myocardial infarction, pulmonary embolism, treatment with thrombolytic therapy | 3,48 | 120 803,676 | ||
| st25.004 | Diagnostic examination of the cardiovascular system | 1,01 | 35 060,837 | ||
| st25.005 | Surgeries on the heart and coronary vessels (level 1) | 2,11 | 73 245,907 | 120 225,11 | |
| st25.006 | Surgeries on the heart and coronary vessels (level 2) | 3,97 | 137 813,389 | ||
| st25.007 | Surgeries on the heart and coronary vessels (level 3) | 4,31 | 149 616,047 | ||
| Heart rhythm disturbances | st13.004 | Rhythm and conduction disorders (level 1) | 1,12 | 38 879,344 | 54 326,94 |
| st13.005 | Rhythm and conduction disorders (level 2) | 2,01 | 69 774,537 | ||
| Stroke | st15.013 | Brain hemorrhage | 2,82 | 97 892,634 | 112 559,17 |
| st15.014 | Cerebral infarction (level 1) | 2,52 | 87 478,524 | ||
| st15.015 | Cerebral infarction (level 2) | 3,12 | 108 306,744 | ||
| st15.016 | Cerebral infarction (level 3) | 4,51 | 156 558,787 | ||
| Angina pectoris | st27.006 | Angina (except unstable), chronic ischemic heart disease (level 1) | 0,78 | 27 076,686 | 58 232,23 |
| st27.007 | Angina (except unstable), chronic ischemic heart disease (level 2) | 1,7 | 59 013,29 | ||
| st13.001 | Unstable angina, myocardial infarction, pulmonary embolism (level 1) | 1,42 | 49 293,454 | ||
| st13.002 | Unstable angina, myocardial infarction, pulmonary embolism (level 2) | 2,81 | 97 545,497 | ||
| Arterial hypertension | st27.005 | Hypertension in the acute stage | 0,7 | 24 299,59 | 37 143,66 |
| st25.004 | Diagnostic examination of the cardiovascular system | 1,01 | 35 060,837 | ||
| st38.001 | Somatic diseases complicated by senile asthenia | 1,5 | 52 070,55 | ||
| CHF | st25.004 | Diagnostic examination of the cardiovascular system | 1,01 | 35 060,837 | 41 916,79 |
| st27.008 | Other heart diseases (level 1) | 0,78 | 27 076,686 | ||
| st27.009 | Other heart diseases (level 2) | 1,54 | 53 459,098 | ||
| st38.001 | Somatic diseases complicated by senile asthenia | 1,5 | 52 070,55 | ||
| Rehabilitation after MI | Providing medical care according to the KSG 350 tariff “Medical cardiac rehabilitation (5 points according to ShRM)” | 64 164,40 | |||
| Rehabilitation after stroke | Providing medical care according to the tariffs of KSG 341-344 “Medical rehabilitation of patients with diseases of the central nervous system (3-6 points according to the ShRM)” - average value of KZ | 119 105,17 | |||
When assessing the costs of CVD treatment, it was taken into account that achieving HbA<7% can reduce the incidence of cardiovascular events [30]. Thus, a reduction in the incidence of all cardiovascular events is expected by 29% (95% CI 0.51-0.98), stroke - by 32% (95% CI 0.46-0.99).
In addition to the direct costs of drug therapy and treatment of exacerbations, indirect costs associated with payment for temporary disability and lost GDP as a result of temporary disability were also taken into account. The incidence of deaths in type 2 diabetes, according to the pharmaco-epidemiological study FORSYTH-DM2, is 40.6 per 100,000 patients or 0.0406%. When assessing the costs of paying for temporary disability, we took into account the median average monthly salary for 2022 of RUB 42,364.00. [31]. In this case, the average daily wage will be 1,412.13 rubles. The assumption was made that payment for temporary disability was carried out at 100%.
When assessing the volume of lost GDP, it was taken into account that in 2022 it was 103,626.60 billion rubles. The working population of Russia in 2018 amounted to 82,264.00 thousand people (56.01%) with a total population of 146,880.00 thousand people. Based on this, GDP per capita of the working population in 2022 amounted to 1,259,683.46 rubles. per year or 3,451.19 rubles. per calendar day. At the same time, the average duration of disability for various cardiovascular events was taken into account: myocardial infarction - 122 days, angina pectoris and chronic heart failure - 15 days each, arterial hypertension and heart rhythm disturbances - 8 days each.
The final value of total costs was calculated taking into account:
- direct costs of comparison insulins and treatment of cardiovascular events (direct medical costs), as well as estimated costs of severe hypoglycemia;
- indirect costs of paying for temporary disability (direct non-medical costs), and costs associated with lost GDP for the period of temporary disability (indirect costs).
Special instructions for the use of the drug Insulin detemir
Insulin detemir provides better glycemic control (based on fasting plasma glucose measurements) compared with isophane insulin. Insufficient insulin dosage or discontinuation of treatment, especially in type 1 diabetes mellitus, can lead to the development of hyperglycemia or diabetic ketoacidosis. Typically, the first symptoms of hyperglycemia appear gradually, over several hours or days. These symptoms include thirst, increased urination, nausea, vomiting, drowsiness, redness and dryness of the skin, dry mouth, loss of appetite, and the smell of acetone in the exhaled air. In type 1 diabetes without appropriate treatment, hyperglycemia leads to the development of diabetic ketoacidosis and can lead to death. Hypoglycemia may occur if the insulin dose is too high in relation to the insulin requirement of a particular patient. Skipping meals or intense exercise can lead to hypoglycemia. After compensation of carbohydrate metabolism, for example, with intensified insulin therapy, patients may experience changes in their typical symptoms that are precursors of hypoglycemia, of which patients should be informed. The usual warning symptoms may disappear with prolonged diabetes mellitus. Concomitant diseases, especially infectious ones and those accompanied by fever, usually increase the body's need for insulin. Transfer from other types of insulin Transfer of a patient to a new type of insulin or insulin from another manufacturer should occur under strict medical supervision. If the concentration, manufacturer, type, type (animal, human, analogues of human insulin) and/or method of its production (genetically engineered or animal-derived insulin) change, dose adjustment may be required. Patients switching to treatment with insulin detemir may require dose changes compared to the doses of previously used insulin. The need for dosage adjustments may occur after the first dose or during the first few weeks or months. Insulin detemir should not be administered intravenously as this may lead to a state of severe hypoglycemia. Absorption with IM administration occurs faster and to a greater extent compared to subcutaneous administration. If insulin detemir is mixed with other types of insulin, the action profile of one or both components will change. Mixing insulin detemir with a rapid-acting insulin analog such as insulin aspart results in an action profile with a reduced and delayed maximum effect compared to administering them separately. Conversion from intermediate-acting and long-acting insulins to insulin levemir may require adjustment of the dose and time of administration. As with other insulins, careful monitoring of blood glucose levels is recommended during the transition and in the first weeks of starting a new insulin. It may be necessary to adjust concomitant hypoglycemic therapy (dose and timing of administration of short-acting types of insulin or dose of oral hypoglycemic agents). Insulin detemir is not intended for use in insulin pumps. Use during pregnancy and lactation. There are currently no clinical data on the use of insulin detemir during pregnancy and lactation. A study of reproductive function in animals did not reveal differences between insulin detemir and human insulin in terms of embryotoxicity and teratogenicity. In general, careful monitoring of pregnant women with diabetes is necessary throughout pregnancy, as well as when planning pregnancy. The need for insulin usually decreases in the first trimester of pregnancy, then increases in the second and third trimesters. Shortly after birth, insulin requirements quickly return to pre-pregnancy levels. In women who are breastfeeding, adjustments to the inulin dose and diet may be required. Impact on the ability to drive a car and operate machinery. Patients' ability to concentrate and react quickly may be impaired during hypoglycemia or hyperglycemia, which can be dangerous in situations where these abilities are especially needed (for example, when driving or operating machinery). Patients should be advised to take measures to prevent the development of hypoglycemia and hyperglycemia when driving a car and working with machinery. This is especially important for patients with the absence or decrease in the severity of symptoms that are warning signs of developing hypoglycemia or frequent episodes of hypoglycemia. In these cases, the advisability of driving or performing similar work should be considered.
Introduction
The events of recent months, which have shaken civilization, again and again force us to think seriously about increasing the effectiveness of treatment of chronic diseases, since these groups of patients are the most vulnerable, including during viral epidemics [1].
Type 2 diabetes mellitus (DM2) is no exception - patients who have not achieved effective control of the disease, assessed by achieving the target level of glycated hemoglobin (HbA1c), experience infectious and other stressful situations in general more severely, the likelihood of a worsening prognosis of the disease increases, Additional treatment measures are required, which also affects public health costs [2]. Effective and safe insulin therapy has always been considered the basis of diabetes control, despite the development and introduction into clinical practice of new classes of antidiabetic drugs over the past 20 years. Our country has extensive experience in the use of both domestic insulin analogs (insulin glargine 100 U/ml and 300 U/ml), which meet the requirements of strict, but at the same time individualized, control of diabetes, and foreign ones (insulin detemir, insulin degludec). Insulin glargine and insulin detemir have been widely used in domestic clinical practice for more than a decade. The paradigm for effective control of type 2 diabetes with these drugs involves timely insulinization in order to prevent micro- and macrovascular complications of the disease and thereby reduce treatment costs [3]. At the same time, different approaches to comparing the dosages of these insulin analogues and to the frequency of their administration have the right to exist [4][5]. Insulin glargine 100 U/ml and insulin detemir do not solve the key problem of insulin therapy - hypoglycemia. In addition, the duration of the hypoglycemic effect of these drugs no longer meets the ever-increasing criteria for ease of use by patients who want to have products with the longest possible duration of action, a minimum volume of administered substance without causing lipodystrophies of the skin, products that do not affect body weight, etc. , which is important for optimizing your lifestyle.
Issues of duration of action, improved safety profile, including during titration, flexibility of prescription (3 hours before/after the usual time of administration) if necessary, are largely resolved with the advent of new analogues into practice: insulin glargine 300 U/ml (Tujeo SoloStar, produced by Sanofi-Aventis Vostok CJSC, Russia) and insulin degludec (Tresiba, produced by JSC Novo Nordisk, Denmark), which, due to the distinctive features of pharmacodynamics and pharmacokinetics, are classified as second-generation drugs [6][7] . This emphasizes their high pharmacological potential for the control of both types of diabetes with a minimum of undesirable effects, primarily hypoglycemic events and a neutral effect on body weight. It is now well known that hypoglycemia is not only a factor in increasing the risk of sudden death by 2 times, hospitalization, and cardiovascular accidents by 30-40%, but also iatrogenic, significantly increasing the costs of the healthcare system for compensation for diabetes [8][9].
An increase in costs in the event of an increase in hypoglycemic events in type 2 diabetes has also been demonstrated for domestic real-life conditions. To a greater extent, this applies to NPH insulin, the frequency of symptomatic and severe hypoglycemia when used for the purpose of effective (to the goal) control of the disease is several times higher compared to insulin glargine 100 U/ml [10]. Costs in groups of patients who use insulin glargine 100 U/ml and NPH insulin differ: despite the higher cost of the insulin analogue (when comparing the cost of unit activity), the costs of controlling type 2 diabetes are generally lower, including for due to fewer hypoglycemia [11]. These examples clearly provide evidence that the low cost per unit of the active substance should not be decisive in the choice of drug for treatment, since what is more important is how much it costs to achieve clinical effectiveness (control of type 2 diabetes), for how long and with what undesirable effects.
Currently, when the main insulin analogues in our country for type 2 diabetes are insulin glargine and insulin detemir, the question of the comparative cost aspects of treating type 2 diabetes with these drugs is being raised with renewed vigor. Considering the introduction of insulin glargine 300 U/ml, we will narrow the question to comparing the economic differences between it and insulin detemir, since it has not yet been studied.
Thus, the purpose of this analysis is to study the comparative cost-effectiveness of insulin glargine 300 U/ml and insulin dete-mir for type 2 diabetes.
Drug interactions Insulin detemir
There are a number of medications that affect the need for insulin. The hypoglycemic effect of insulin is enhanced by: oral hypoglycemic drugs, MAO inhibitors, ACE inhibitors, carbonic anhydrase inhibitors, non-selective β-blockers, bromocriptine, sulfonamides, anabolic steroids, tetracyclines, clofibrate, ketoconazole, mebendazole, pyridoxine, theophylline, cyclophosphamide, fenfluramine, lithium preparations, drugs containing ethanol. The hypoglycemic effect of insulin is weakened by: oral contraceptives, corticosteroids, thyroid hormones, thiazide diuretics, heparin, tricyclic antidepressants, sympathomimetics, danazol, clonidine, slow calcium channel blockers, diazoxide, morphine, phenytoin, nicotine. Under the influence of reserpine and salicylates, it is possible to either weaken or enhance the effect of the drug Octreotide/lanreotide, which can either increase or decrease the body’s need for insulin. β-adrenergic blockers may mask symptoms of hypoglycemia and delay recovery from hypoglycemia. Alcohol can enhance and prolong the hypoglycemic effect of insulin. Incompatibility Some drugs, for example those containing thiol or sulfite, when added to insulin detemir solution, can cause its destruction. Therefore, insulin detemir should not be added to infusion solutions.
Overdose of the drug Insulin detemir, symptoms and treatment
There is no specific dose that allows us to talk about an insulin overdose, but hypoglycemia can develop gradually if the dose was administered too high for a particular patient. Symptoms of hypoglycemia. Treatment: the patient can eliminate mild hypoglycemia himself by ingesting glucose, sugar or carbohydrate-rich foods. Therefore, patients with diabetes are advised to carry sugar, sweets, cookies or sweet fruit juice with them at all times. In case of severe hypoglycemia, when the patient is unconscious, 0.5–1 mg of glucagon should be administered IM or SC (can be administered by a trained person), or IV dextrose (glucose) solution; INSERT INTO `info` (`ID`, `Name`, `NameBase`, `TEXT`, `IsUsed`, `Description`, `KeyWords`) VALUES (can only be entered by a medical professional). It is also necessary to administer intravenous dextrose if the patient does not regain consciousness 10–15 minutes after the administration of glucagon. After regaining consciousness, the patient is advised to eat a meal rich in carbohydrates to prevent relapse of hypoglycemia.
List of pharmacies where you can buy Insulin detemir:
- Moscow
- Saint Petersburg