Detailed description of the study
Levetiracetam is an antiepileptic drug. It differs from other drugs in this group in its high efficiency and fewer side effects. Analysis for levetiracetam allows you to identify the cause of treatment failure and adjust the dose of the drug.
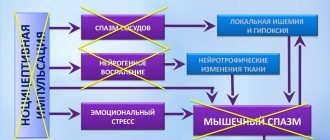
Epilepsy is a brain disease characterized by abnormal electrical activity in neurons, leading to seizures. The impulse between the cells of the nervous system is transmitted through special synapses. During impulse transmission, special substances are released, the vesicles of which contain inhibitory molecules, such as gamma-aminobutyric acid (GABA), or excitatory molecules. Calcium also influences intercellular interaction, which promotes arousal. Levetiracetam inhibits the influx of Ca. A feature of the mechanism of action of the drug is binding to a special protein of synaptic vesicles, which ensures the proper functioning of neurons.
Epilepsy is divided into generalized and partial - with established localization in the brain. Levetiracetam is effective in stopping attacks in both forms. There is idiopathic epilepsy, the causes of which cannot be determined, it is associated with genetic factors, there is no previous brain damage and begins mainly in childhood.
Symptomatic is associated with visible causes: organic lesions of the central nervous system, previous infections (meningitis, encephalitis), high body temperature, hypoxia, and can occur at any age.
There are many types of seizures, but they are all characterized by a sudden onset and spontaneous cessation, short duration and similarity throughout the illness. Symptomatic epilepsy is most often accompanied by mental disorders: impaired attention, memory; the occurrence of anxiety, depression.
Levetiracetam is effective for the treatment of partial seizures with or without generalization, particularly for the treatment of juvenile myoclonic epilepsy. This form is quite common and is characterized by the onset of the disease in adolescence (from 12 years), presumably due to a genetic predisposition. Symptoms are involuntary muscle contractions (in the arms) and tonic convulsions of the whole body with loss of consciousness. Most often associated with sleep disturbances (insomnia). This form usually does not lead to mental disorders.
Levetiracetam may cause side effects. These include: weakness, drowsiness, mood swings, emotional instability, weight loss, nausea, abdominal pain. A skin rash, itching, and muscle pain may also appear. The drug has an effect on the health of elderly patients and people with liver and kidney diseases.
Levetiracetam does not require constant monitoring of concentrations in blood tests like other antiepileptic drugs, such as carbamazepine. But periodic determination allows you to avoid side effects, thereby improving a person’s health and quality of life.
Levetiracetam
Levetiracetam is a highly soluble and permeable compound. The pharmacokinetic profile is linear with low intra- and interindividual variability. After long-term use, there is no change in clearance. There is no evidence of sex, race, or diurnal differences. The pharmacokinetic properties of levetiracetam in patients with epilepsy and healthy volunteers are comparable.
Due to complete and linear absorption, plasma concentrations can be predicted from the dose of levetiracetam expressed in mg/kg body weight. Therefore, it is not necessary to monitor plasma concentrations of levetiracetam.
In adults and children, a high correlation is shown between the concentration of levetiracetam in plasma and saliva (saliva/plasma ratio ranges from 1-1.7 for oral tablets and for oral solution four hours after taking the latter).
Adults and teenagers
Absorption
After oral administration, levetiracetam is rapidly absorbed. Absolute bioavailability after oral administration is close to 100%.
The maximum concentration in plasma (Cmax) is achieved after 1.3 hours. The equilibrium state is achieved after two days when taking the drug twice a day.
Cmax is usually 31 and 43 mcg/ml after a single dose of 1000 mg and a dose of 1000 mg twice a day, respectively.
The amount of absorption does not depend on the dose or food intake.
Distribution
There are no data on distribution in humans. Levetiracetam and its main metabolite are weakly bound to plasma proteins (<10%). The volume of distribution of levetiracetam is about 0.5-0.7 l/kg, which approximately corresponds to the volume of water in the body.
Biotransformation
Levetiracetam is poorly metabolized in the human body. The main metabolic pathway (24% of the dose) is enzymatic hydrolysis of the acetamide group. Liver cytochrome P450 isoenzymes are not involved in the formation of the main metabolite (ucb L057). Hydrolysis of the acetamide group occurs in many tissues, including blood cells. Metabolite ucb L057 is pharmacologically inactive.
Two minor metabolites were also detected. The first is formed due to hydroxylation of the pyrrolidone ring (1.6% of the dose), the second - by opening of the pyrrolidone ring (0.9% of the dose).
Other unidentified metabolites account for only 0.6% of the dose. Optical isomerization of levetiracetam and its main metabolite in vivo has not been detected.
Levetiracetam and its main metabolite do not inhibit the main human liver cytochrome P450 isoenzymes (CYP3A4, 2A6, 2C9, 2C19, 2D6, 2E1 and 1A2), glucuronyl transferase (UGT1A1 and UGT1A6) and epoxide hydroxylase in vitro. Levetiracetam also does not affect the glucuronidation of valproic acid in vitro.
In cultured human hepatocytes, levetiracetam had little or no effect on the activity of the CYP1 A2, SULT1E1 and UGT1A1 isoenzymes. Levetiracetam weakly induced the activity of the CYP2B6 and CYP3A4 isoenzymes.
In vitro data and in vivo drug interaction data with oral contraceptives, digoxin, and warfarin indicate that significant enzyme induction is not expected in vivo. Therefore, interaction of levetiracetam with other substances is unlikely.
Removal
The half-life in adults is 7±1 hours and is independent of dose, route of administration or duration of use. The average total clearance is 0.96 ml/min/kg.
The main route of elimination is excretion in the urine (about 95% of the dose, of which 93% is excreted within 48 hours). Excretion in feces is only 0.3% of the dose.
The total excretion of levetiracetam and its main metabolite is 66 and 24% of the administered dose, respectively, during the first 48 hours. The renal clearance of levetiracetam and ucb L057 is 0.6 and 4.2 ml/min/kg, respectively, indicating excretion of levetiracetam through glomerular filtration with subsequent tubular reabsorption of the main metabolite by, along with glomerular filtration, active tubular secretion.
Elimination of levetiracetam correlates with creatinine clearance.
Elderly
The half-life in the elderly increases by 40% (up to 10-11 hours), which is due to a decrease in renal function in this population group.
Kidney failure
The apparent clearance of levetiracetam and its main metabolite depends on creatinine clearance. In this regard, in patients with moderate and severe renal failure, it is recommended to adjust the maintenance dose of the drug depending on creatinine clearance.
In adult patients with end-stage renal failure, the half-life is 25 hours between hemodialysis sessions and 3.1 hours during the procedure itself.
During a typical four-hour hemodialysis session, about 51% of levetiracetam is removed.
Liver dysfunction
In patients with mild to moderate hepatic impairment, the clearance of levetiracetam changes slightly. In most patients with severe hepatic impairment, levetiracetam clearance is reduced by more than 50% due to concomitant renal impairment.
Children under 12 years old
Children aged 4-12 years
After a single dose of 20 mg/kg, the half-life in children 6-12 years of age is 6 hours. Body weight-adjusted apparent clearance is 30% higher than that in adults with epilepsy.
After long-term administration of the drug at a dose of 20-60 mg/kg/day, absorption of levetiracetam in children 4-12 years old is rapid; Cmax is reached within 0.5-1 hour. Cmax and the area under the concentration-time curve are linear and proportional to the dose. The terminal half-life is 5 hours. The apparent clearance is 1.1 ml/min/kg.
References
- Romanenko, I.V., Romanenko, V.I., Romanenko, Yu.I. Antiepileptic, neuroprotective and antihyperalgic effects of levetiracetam. International Neurological Journal, 2017. - No. 5(91). — P. 68-72.
- Diagnosis and treatment of mental disorders in epilepsy. Clinical recommendations. Russian Society of Psychiatrists, 2014. - 36 p.
- Mukhin, K.Yu., Freidkova, N.V., Glukhova, L.Yu. et al. Juvenile myoclonic epilepsy: focus on the effectiveness of therapy and relapse rate according to long-term follow-up data. Russian Journal of Child Neurology, 2015. - T. 10(4). — P. 7-16.
- Kolyagin, V.V. Etiology, classification and treatment of epilepsy. Irkutsk: RIO IGIUVA, 2010. - 40 p.
- Vidal reference book of medicines: website / Levetiracetam, 2022. - URL: https://www.vidal.ru/drugs/levetiracetam__33975 (access date: 08/12/2021).
Relevance
Valproate is the first-line treatment for patients with newly diagnosed idiopathic generalized or difficult-to-classify epilepsy, excluding women of childbearing age due to teratogenicity.
Recently, there has been an increase in the frequency of prescription of levetiracetam in patients with this type of epilepsy without scientifically proven clinical or economic effectiveness.
results
- The final analysis included 520 patients randomized 1:1 to valproate or levetiracetam.
- The average follow-up period was 2 years. The average age of study participants was 13.9 years (65% boys and men). Most patients were very concerned about generalized epilepsy.
- In the intention-to-treat analysis, levetiracetam did not meet the non-inferiority criterion for time to 12-month remission (hazard ratio, 1.19 [95% CI 0.96 to 1.47).
- In a per-protocole analysis, the 12-month remission rate was higher with valproate compared with levetiracetam.
- According to the safety analysis, adverse events were observed in 37% of patients in the valproate group and in 42% of patients in the levetiracetam group.
- It was shown that levetiracetam was not superior to valproate in terms of cost-effectiveness.
Methods
An open-label randomized controlled trial was performed comparing levetiracetam with valproate as first-line treatment in patients with newly diagnosed idiopathic generalized or unclassified epilepsy.
Study enrollment took place at 69 UK sites. Study participants were patients aged 5 years and older.
For patients aged 12 years and older, the maintenance dose of valproate and levetiracetam was 500 mg twice daily. For children aged 5-12 years, the dose of valproate was 25 mg/kg, the dose of levetiracetam was 40 mg/kg.
As a featherbed end point
time to 12-month remission was considered. As a non-inferiority criterion, a risk ratio of 1.314 was chosen, corresponding to an absolute difference of 10%.