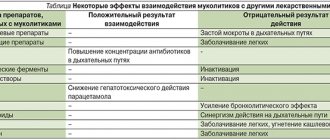
Orniona, 0.1%, vaginal cream, 15 g, 1 pc.
Atrophy of the mucous membrane of the lower genitourinary tract, caused by estrogen deficiency, can be manifested by unpleasant sensations in the vagina (pain during intercourse, vaginal dryness, itching) and urinary disorders (increased frequency of urination, dysuria, mild urinary incontinence).
To treat postmenopausal symptoms, HRT should only be started for symptoms that adversely affect quality of life. In all cases, a thorough assessment of the risks and benefits of treatment should be carried out at least once a year. HRT should only be continued for a period of time when the benefit outweighs the risk.
There is limited evidence of an increased risk with HRT during treatment of premature menopause. Because younger women have a lower absolute risk, their benefit-risk ratio is more favorable than that of older women.
Medical examination/observation
Before starting or resuming HRT, a detailed individual and family history should be obtained. Based on the medical history, contraindications and warnings for the use of the drug, it is necessary to conduct a clinical examination, including examination of the pelvic organs and mammary glands.
During treatment, it is recommended to conduct a general medical and gynecological examination, including examination of the mammary glands. The frequency and nature of examinations are individual, but at least once a year. Women should be informed about the need to inform their doctor about changes in the mammary glands. Investigations, including appropriate imaging techniques such as mammography, should be performed in accordance with generally accepted examination standards and on a case-by-case basis.
Therapy should be discontinued if a contraindication is identified and/or if the following conditions/diseases occur:
- jaundice and/or deterioration of liver function;
- significant increase in blood pressure;
- occurrence or resumption of migraine-type headaches;
- pregnancy.
Endometrial hyperplasia and endometrial cancer
To prevent endometrial stimulation, the daily dose of Orniona® should not exceed 1 administration (0.5 mg estriol), and this dose should not be used daily for more than 4 weeks in a row. Vaginal bleeding in all cases requires examination. The patient should be informed of the need to contact the attending physician if vaginal bleeding begins.
Mammary cancer
HRT may increase mammographic density. This can complicate the X-ray diagnosis of breast cancer. Clinical studies have shown that the likelihood of an increase in mammographic density is lower in women treated with estriol than in women treated with other estrogens.
Overall evidence suggests an increased risk of developing breast cancer in women receiving combination therapy with estrogens and progestogens and possibly estrogen monotherapy.
In women receiving combination therapy with estrogens and progestogens for more than 5 years, a 2-fold increase in the risk of breast cancer was noted.
With estrogen monotherapy, the increase in the risk of developing breast cancer is significantly lower than when combined with progestogens. The level of risk depends on the duration of HRT.
Ovarian cancer
Ovarian cancer develops much less frequently than breast cancer. Long-term estrogen monotherapy (at least 5–10 years) was associated with a small increase in the risk of ovarian cancer. Some studies suggest that combined HRT may increase the risk of ovarian cancer in a similar or small way. It is not known whether the risk of long-term use of low-dose estrogens (such as Orniona®) differs from that of monotherapy with other estrogens.
VTE
HRT is associated with an increased risk of developing VTE, i.e. deep vein thrombosis or pulmonary embolism, 1.3–3 times. The likelihood of developing VTE during the first year of HRT use is higher than at a later date. This risk is unknown for Orniona®.
In patients with a congenital or acquired predisposition to the development of arterial or venous thrombosis, the risk of VTE is high, and HRT may further increase it. In this regard, HRT is contraindicated for such patients (see “Contraindications”).
Generally recognized risk factors for VTE include estrogen use, older age, major surgery, prolonged immobilization, obesity (BMI greater than 30 kg/m2), pregnancy/puerperium, systemic lupus erythematosus, and cancer. There is no consensus regarding the possible role of varicose veins in the development of VTE. After any surgical intervention, VTE prophylaxis is necessary. If prolonged immobilization is associated with elective surgery, it is necessary to temporarily discontinue HRT 4–6 weeks before surgery. Treatment should be resumed after the patient begins to walk.
For patients already receiving anticoagulant treatment, careful consideration of the benefit-risk ratio of HRT is required.
If Orniona® is prescribed as pre- and postoperative treatment, the issue of thrombosis prophylaxis should be considered.
In the absence of a history of VTE in the patient, but in the presence of thrombosis at a young age in her immediate family, the patient can be offered a screening examination, having previously discussed all its limitations (screening can detect only a number of hemostasis disorders). If a hemostasis defect is detected that does not correspond to the disease in relatives, or if a severe defect is detected (for example, deficiency of antithrombin III, protein S or protein C, or a combination of these defects), HRT is contraindicated.
If VTE develops after starting treatment with Orniona®, treatment should be discontinued. The patient should be informed to seek immediate medical attention if she experiences possible signs of VTE (eg, painful swelling of the lower extremity, sudden chest pain, shortness of breath).
IHD
There are no results from randomized controlled trials that indicate that combination therapy with estrogens and progestogens and estrogen monotherapy can prevent the development of myocardial infarction in women with or without coronary artery disease.
According to randomized controlled trials, in patients with a removed uterus, the risk of coronary artery disease did not increase with estrogen monotherapy.
The risk of coronary artery disease increases slightly with combined HRT with estrogens and progestogens in patients over 60 years of age.
Ischemic stroke
Combination therapy with estrogens and progestogens and monotherapy with estrogens are associated with a 1.5-fold increase in the risk of ischemic stroke. The relative risk does not vary with age or time since menopause. However, the baseline risk of stroke is largely dependent on age, and the overall risk of stroke with HRT increases with age.
The risk of hemorrhagic stroke does not increase with HRT.
Other states
Estrogens can cause fluid retention, and therefore patients with impaired renal function and cardiovascular insufficiency should be closely monitored by a physician.
With initial hypertriglyceridemia, the concentration of triglycerides in the blood plasma may increase during HRT, which may result in the development of pancreatitis.
Estriol is a weak gonadotropin inhibitor and has no other significant effects on the endocrine system.
HRT does not improve cognitive function. Evidence was obtained of an increased risk of developing dementia in patients who began using combination therapy or monotherapy in a continuous regimen after 65 years.
In the presence of vaginal infections, concomitant specific treatment is recommended.
Orniona® contains cetyl alcohol and stearyl alcohol, which can cause local skin reactions (for example, contact dermatitis).
Impact on the ability to drive vehicles and operate machinery.
Estriol does not affect coordination of movements, concentration and the ability to drive vehicles and other mechanisms.
Orniona®
HRT for the treatment of symptoms of estrogen deficiency should be carried out only for symptoms that adversely affect the woman’s quality of life. At least once a year, a thorough assessment of the benefit-risk ratio should be carried out; continuation of therapy is justified only if the benefit of using the drug exceeds the risk. There is limited evidence of the risks associated with HRT when treating premature menopause. Due to the low absolute risk in younger women, their benefit-risk ratio is more favorable than in older women.
Medical examination/observation
Before starting or resuming HRT after its interruption, it is necessary to collect a detailed individual and family history, conduct a general and gynecological examination (including examination of the mammary glands and pelvic organs). During the therapy period, it is recommended to conduct periodic medical examinations, the frequency and nature of which are determined individually. The woman should be informed about the need to inform the doctor about possible changes in the mammary glands. Screenings, including appropriate imaging modalities such as mammography, should be performed according to currently accepted screening standards and on a case-by-case basis.
Reasons for immediate discontinuation of therapy
Therapy should be stopped immediately if contraindications are identified and if the following conditions occur:
- jaundice or deterioration of liver function;
- significant increase in blood pressure;
- the occurrence of migraine-type headaches;
- pregnancy.
Hyperplasia and endometrial cancer
To prevent stimulation of the endometrium, the daily dose of estriol should not exceed one administration (0.5 mg of estriol) per day. This maximum dose should not be used for more than 4 weeks. One epidemiological study found that long-term, low-dose oral estriol may increase the risk of endometrial cancer. The risk increases with the duration of treatment and returns to baseline values one year after discontinuation of the drug. Basically, the risk of developing minimally invasive and highly differentiated tumors increases. If spotting/bleeding from the vagina occurs, an appropriate examination is necessary. The patient should be informed of the need to contact the attending physician if bleeding begins.
Mammary cancer
Long-term use of HRT increases the risk of developing breast cancer in women receiving combination therapy with estrogen and progestogen and, possibly, estrogen monotherapy. In women receiving combined estrogen + progestogen therapy for more than 5 years, there was a 2-fold increase in the risk of developing breast cancer. With estrogen monotherapy, the increase in risk is significantly lower than when combined with a progestogen. Limited data indicate that there is no risk of developing breast cancer with the use of estriol.
HRT, particularly combination drugs, may increase the density of mammographic images. This may complicate radiological detection of breast cancer.
Ovarian cancer
Ovarian cancer develops much less frequently than breast cancer. Long-term estrogen monotherapy (for at least 5 to 10 years) was associated with a small increase in the risk of ovarian cancer. Some studies suggest that HRT with combination drugs has a similar or slightly lower risk. It is not known whether the risk of long-term use of low-potency estrogens (such as estriol) is different from that of monotherapy with other estrogens.
VTE
HRT is associated with an increase in the risk of developing VTE (deep vein thrombosis or pulmonary embolism) by 1.3-3 times. The likelihood of developing VTE is higher during the first year of HRT use than at a later date. In patients with a confirmed thrombophilic condition, the risk of VTE is high, and HRT may further increase this risk. Therefore, HRT is contraindicated in such women.
Generally recognized risk factors for VTE include estrogen use, older age, major surgery, prolonged immobilization, obesity (BMI >30 kg/m2), pregnancy/puerperium, systemic lupus erythematosus, and cancer. There is no consensus regarding the possible role of varicose veins in the development of VTE. After any surgical intervention, VTE prophylaxis is necessary. In case of prolonged immobilization due to planned surgery, HRT should be temporarily discontinued 4-6 weeks before surgery and resumed only after the woman has regained full mobility.
In the absence of a woman's history of VTE, but in the presence of thrombosis at the age of less than 50 years in close relatives, it is recommended to conduct a screening examination, having previously discussed all its limitations (screening can only identify a number of thrombophilic disorders). If a disorder is detected that does not correspond to the disease in relatives, or if a “severe” defect is detected (for example, deficiency of antithrombin III, protein S or protein C, or a combination of these defects), HRT with estriol is contraindicated.
For women receiving long-term anticoagulant treatment, careful consideration of the benefit-risk profile of HRT is required.
If VET develops, therapy with Orniona should be discontinued immediately. A woman should be informed of the need to immediately consult a doctor if possible signs of thromboembolic complications appear (for example, swelling or tenderness along the vein of the lower extremity, sudden chest pain, shortness of breath, etc.).
IHD
In randomized controlled clinical trials, there was no evidence that HRT with combination drugs or estrogen monotherapy can prevent the development of myocardial infarction in women with and without coronary artery disease.
Estrogen monotherapy
According to randomized controlled clinical trials, in women with a history of hysterectomy, the risk of coronary artery disease is not increased with estrogen monotherapy. The absolute risk of coronary heart disease increases slightly with HRT with combined (estrogen + gestagen) drugs in patients over 60 years of age.
Ischemic stroke
HRT with combination drugs and estrogen monotherapy are associated with a 1.5-fold increase in the risk of ischemic stroke. The relative risk does not change with age and time after menopause. However, the baseline risk of stroke depends to a large extent on age, and accordingly, the overall risk of ischemic stroke during HRT increases with age. The risk of hemorrhagic stroke does not increase with HRT.
Other states
Estrogens can cause fluid retention, and therefore patients with chronic heart and kidney failure should be under close medical supervision.
Estriol is a weak gonadotropin antagonist and has no other significant effects on the endocrine system.
It is necessary to monitor lipid levels in patients with pre-existing hypertriglyceridemia because, in rare cases, estrogen HRT has caused a significant increase in plasma triglyceride concentrations, leading to the development of pancreatitis.
Estrogens cause an increase in the concentration of thyroxine-binding globulin, which leads to an increase in total circulating thyroid hormone, measured as total protein-bound iodine; T4 (thyroxine) concentration or T3 (triiodothyronine) concentration.
The concentration of other plasma proteins (angiotensin-renin substrate, alpha-1-antitrypsin, ceruloplasmin) may also increase.
Cognitive function does not improve with HRT. There is evidence of an increased risk of developing dementia in women who start HRT with combination drugs or continuous estrogen monotherapy after 65 years.
Orniona® contains cetyl alcohol and stearic alcohol, which can cause local skin reactions (for example, contact dermatitis).