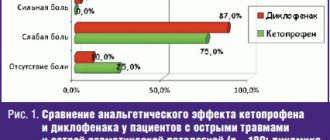
Release form
The medicine is produced in the form of Ketoprofen gel, which has a uniform consistency, transparent or light yellow color, intended for external use. In addition, there are subtypes of this drug in the form of tablets, suppositories, as well as a solution for intramuscular and intravenous administration.
2.5% gel is packaged in specialized tubes made of aluminum foil or polyethylene, 50 grams each, and placed in cardboard packages of 1 piece.
The drug is not produced in the form of an ointment.
Pharmacodynamics and pharmacokinetics
When used, Ketoprofen gel exhibits its analgesic and anti-inflammatory properties. The active medicinal component included in the drug helps suppress 1, 2 cyclooxygenase activity , ultimately regulating the synthesis of prostaglandins.
When using the gel in the treatment of joint syndrome, it is possible to significantly reduce the pain experienced by patients, both during movement and at rest, as well as reduce swelling of the joints and eliminate their morning stiffness. During local use, the ointment is absorbed rather slowly and does not accumulate in the human body.
The bioavailability of Ketoprofen is at the level of 5%. The drug does not form active metabolites is metabolized by conjugation with glucuronic acid.
Indications for use
What is Ketoprofen effective for? The drug is indicated for use for the following ailments and diseases:
- diseases of the musculoskeletal system, for example, rheumatism, articular syndrome, gout, arthritis (rheumatoid, psoriatic types), osteoarthritis, ankylosing spondylitis, osteochondrosis, radiculitis, sciatica, inflammatory tendon and ligament damage, lumbago;
- muscle pain of non-rheumatic and rheumatic origin;
- inflammation of soft tissues of a post-traumatic nature, for example: ruptures, bruises and other damage to the ligaments and musculoskeletal system.
Ketoprofen gel for external use 2.5% 30g
Compound
Active substance: ketoprofen 2.5 g.
Excipients: carbomer - 1.5 g, ethanol 95% - 32 g, trolamine - 2.9 g, lavender oil - 0.1 g, water - up to 100 g.
Pharmacokinetics
Ketoprofen is absorbed very slowly and practically does not accumulate in the body. Bioavailability is 5%. Ketoprofen penetrates into the subcutaneous tissue, ligaments and muscles, synovial fluid and reaches therapeutic concentrations there. The concentration of the drug in the blood plasma is extremely low. Ketoprofen is metabolized in the liver to form conjugates, which are mainly excreted in the urine. Ketoprofen is characterized by slow excretion in urine.
Indications for use
- Acute and chronic inflammatory diseases of the musculoskeletal system (articular syndrome with exacerbation of gout, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, osteoarthritis, osteochondrosis with radicular syndrome, radiculitis, inflammatory damage to ligaments and tendons, bursitis, sciatica, lumbago);
- muscle pain of rheumatic and non-rheumatic origin;
- post-traumatic inflammation of soft tissues and the musculoskeletal system (damage and rupture of ligaments, bruises).
The drug is intended for symptomatic therapy, reducing pain and inflammation at the time of use, and does not affect the progression of the disease.
Contraindications
- Individual hypersensitivity to ketoprofen or other components of the drug, acetylsalicylic acid or other NSAIDs (history of bronchospasm, urticaria or rhinitis caused by taking acetylsalicylic acid), tiaprofenic acid and fenofibrate;
- complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose or paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including a history);
- a history of increased skin sensitivity to solar radiation (photosensitivity);
- history of skin allergies to sunscreens or perfumes;
- exposure to the sun on the treated areas, including solariums, during the course of using the drug and 2 weeks after;
- weeping dermatoses, eczema, infected abrasions, wounds (at the site of application of the gel);
- children under 12 years of age (efficacy and safety have not been studied);
- pregnancy (III trimester) and breastfeeding period.
With caution:
Hepatic porphyria (exacerbation), erosive and ulcerative lesions of the gastrointestinal tract, severe dysfunction of the liver and kidneys, chronic heart failure, bronchial asthma, old age, pregnancy (I and II trimester).
Directions for use and doses
For external use.
A strip of gel 5-10 cm long is applied in a thin layer to the affected area or skin over the source of inflammation 2 times a day and rubbed in lightly.
The amount of gel applied depends on the size of the area being treated.
It is possible to use ketoprofen in combination with physiotherapy (phonophoresis and iontophoresis).
The dosage in children over 12 years of age corresponds to that in adults and depends on the area of application and the doctor’s recommendations.
Storage conditions
In a dark place at a temperature not exceeding 25° C. Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date.
special instructions
Do not apply to damaged (including open wounds) and inflamed skin!
Avoid contact with eyes (risk of conjunctival irritation).
It is recommended to wash your hands thoroughly after using the drug.
Do not use as hermetic dressings. Do not use in combination with sealed clothing.
Long-term use of topical agents may result in sensitization or local irritation.
To avoid any manifestations of hypersensitivity or photosensitivity, exposure to direct sunlight (including visiting a solarium) should be avoided during the treatment period and for two weeks after using the drug, and it is recommended to cover the treated areas with clothing. Patients with severe renal, cardiac or hepatic impairment should be careful when using ketoprofen.
You should stop using the drug if any skin reaction occurs, including reactions when simultaneously applying sunscreen or other cosmetics containing the organic sunscreen filter octocrylene to the skin.
When using the drug in large quantities, it is possible, in very rare cases, to develop systemic side effects (hypersensitivity, bronchial asthma, gastrointestinal disorders, aggravation of renal failure).
The risk of systemic side effects increases depending on the amount of gel applied, the area of the skin being treated, the condition of the skin, and the duration of treatment.
Avoid exposure of treated areas to sunlight or UV rays, including tanning beds, during treatment and for two weeks after treatment.
Description
Non-steroidal anti-inflammatory drug (NSAID).
Use in children
Contraindicated in children under 12 years of age (efficacy and safety have not been studied).
Pharmacodynamics
NSAIDs. It has analgesic, anti-inflammatory and anti-edema effects. Inhibits the activity of COX, which leads to inhibition of prostaglandin synthesis. In addition, ketoprofen inhibits lipoxygenase, bradykinin synthesis, stabilizes lysosomal membranes and prevents the release of enzymes involved in the inflammatory process.
Ketoprofen does not have a negative effect on the condition of articular cartilage.
Side effects
Possible side effects are listed in descending frequency of occurrence: very common (> 10%), common (> 1%, < 10%), uncommon (> 0.1%, < 1%), rare (> 0.01%, < 0.1%), very rare (<0.01%).
From the skin: infrequently - erythema, itching, eczema; rarely - photosensitivity, bullous dermatitis, urticaria; very rarely - contact dermatitis, angioedema.
From the gastrointestinal tract: very rarely - peptic ulcer, bleeding, diarrhea.
From the immune system: very rarely - anaphylactic reactions, hypersensitivity reactions.
From the urinary system: very rarely - aggravation of renal failure.
Use during pregnancy and breastfeeding
The use of the drug during breastfeeding is not recommended.
Interaction
When ketoprofen is used externally in the form of a gel, it is possible to enhance the effect of drugs that cause photosensitivity. Other interactions have not been established, however, patients taking coumarin anticoagulants are recommended to regularly monitor the international normalized ratio (INR).
Overdose
The extremely low systemic absorption of the active components of the drug when used externally makes overdose almost impossible.
If large quantities of the drug (more than 20 g) are accidentally ingested, systemic adverse reactions characteristic of NSAIDs may occur. It is necessary to lavage the stomach and take activated charcoal. Treatment is symptomatic.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive a car or use other mechanical means.
Instructions for use of Ketoprofen (Method and dosage)
Ketoprofen gel, instructions for use
Use only externally, i.e. Apply to the skin with light massaging movements with a strip of about 4-6 cm in the area of inflammation and pain. The course of treatment with the drug (without prior medical consultation and examination) should not exceed 10 days.
Ketoprofen tablets, instructions for use
The dosage of the drug should be determined by the attending physician individually in each specific case. The initial recommended daily dose of the drug for adult patients is 300 mg. Ketoprofen is taken a maximum of 3 times a day.
When taking the drug orally, side effects may occur such as: nausea, constipation, vomiting or diarrhea, gastralgia, pain in the epigastric region, headaches, impaired renal and liver function, dizziness, allergic reactions, tinnitus, ulcerative or erosive character.
Ketoprofen suppositories, instructions for use
The recommended daily dosage when using rectal suppositories should not exceed 300 mg. It is contraindicated to use suppositories in the presence of bleeding from the rectum, as well as proctitis (even in history).
Injections (Ketoprofen solution for intravenous or intramuscular administration) are used for emergency relief of chronic exacerbations, as well as for the treatment of acute conditions in a single dosage not exceeding 100 mg. As a rule, patients are subsequently treated with other forms of the drug.
Ketoprofen gel
Dosage form
Gel for external use
Composition per 100 g:
Active substance
: ketoprofen – 2.5 g.
Excipients
: carbomer – 1.5 g, alcohol 95% – 32.0 g, trolamine – 2.9 g, lavender oil – 0.1 g, purified water – up to 100.0 g.
Description
Colorless, transparent or opalescent gel with a characteristic odor.
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drug (NSAID).
ATX code: M02AA10.
Pharmacological properties
Pharmacodynamics
Ketoprofen is one of the most effective cyclooxygenase inhibitors. It also inhibits lipoxygenase and bradykinin activity. Stabilizes lysosomal membranes and prevents the release of enzymes involved in the inflammatory process. The main properties of ketoprofen are analgesic, anti-inflammatory and anti-edema effects. Ketoprofen does not have a negative effect on the condition of articular cartilage.
Pharmacokinetics
Ketoprofen, when applied topically in the form of a gel, does not accumulate in the body. The bioavailability of the gel is about 5%. The maximum concentration of ketoprofen in the blood plasma is achieved 6 hours after application of the drug. Penetrates into joint tissues, incl. into the synovial fluid, and reaches therapeutic concentrations there. The concentration of the drug in the blood plasma is extremely low.
Ketoprofen is metabolized in the liver to form conjugates, which are excreted primarily by the kidneys. The metabolism of ketoprofen does not depend on age, the presence of severe renal failure or liver cirrhosis. Excretion of ketoprofen by the kidneys is slow.
Indications for use
Symptomatic therapy – reduction of pain and inflammation at the time of use, does not affect the progression of the disease – in the following conditions:
• reactive arthritis (Reiter's syndrome);
• osteoarthritis of various localizations;
• periarthritis, tendinitis, bursitis, myalgia, neuralgia, radiculitis;
• injuries of the musculoskeletal system (including sports), bruises of muscles and ligaments, sprains, ruptures of ligaments and tendons.
Contraindications
• Hypersensitivity to ketoprofen or other components of the drug, as well as to salicylates, tiaprofenic acid or other NSAIDs, fenofibrate; history of skin allergies to sunscreens and perfumes;
• complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses and intolerance to acetylsalicylic acid or other NSAIDs (including a history);
• III trimester of pregnancy;
• children's age (up to 15 years);
• violation of the integrity of the skin in the area where the gel is applied (eczema, acne, weeping dermatitis, open or infected wound);
• history of photosensitivity reactions;
• exposure to sunlight, incl. indirect sunlight and UV irradiation in a solarium throughout the entire treatment period and for another 2 weeks after stopping treatment with the drug.
Carefully
You should consult your doctor before using Ketoprofen gel if you have:
• impaired liver and/or kidney function;
• erosive and ulcerative lesions of the gastrointestinal tract; • blood diseases;
• bronchial asthma;
• chronic heart failure;
• hepatic porphyria (exacerbation).
Use during pregnancy and breastfeeding
Pregnancy
Use in
the 1st and 2nd trimesters of pregnancy
Since the safety of ketoprofen in pregnant women has not been assessed, the use of ketoprofen should be avoided in the 1st and 2nd trimesters of pregnancy.
Use in the third trimester of pregnancy
The drug Ketoprofen gel is contraindicated in the third trimester of pregnancy.
During the third trimester of pregnancy, all prostaglandin synthetase inhibitors, including ketoprofen, can have a toxic effect on the heart, lungs and kidneys of the fetus. At the end of pregnancy, bleeding time in the mother and child may increase. NSAIDs may delay the onset of labor.
Breast-feeding
To date, there is no data on the release of ketoprofen into breast milk, therefore the use of the drug Ketoprofen gel during breastfeeding is not recommended.
Directions for use and doses
For external use.
A small amount of gel (3-5 cm) is applied in a thin layer to the skin of the inflamed or painful area of the body 1-2 times a day and rubbed in gently.
The dosage should be selected in accordance with the area of the affected area: 5 cm of Ketoprofen gel corresponds to 100 mg of ketoprofen, 10 cm corresponds to 200 mg of ketoprofen.
If necessary, Ketoprofen gel can be combined with other dosage forms of the drug Ketoprofen (capsules, tablets, rectal suppositories, solution for intramuscular administration).
The maximum dose of ketoprofen is 200 mg/day.
Occlusive dressing is not recommended.
Do not use without consulting a doctor for more than 14 days.
If you forget to apply the gel, apply it at the time the next dose is due, but do not double it.
Side effect
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (> 1/10), common (> 1/100 to < 1/10), uncommon (> 1/1000 to <1/100), rare (from >1/10000 to <1/1000), very rare (<1/10000); frequency unknown - based on available data, it was not possible to determine the frequency of occurrence.
Immune system disorders
frequency unknown: anaphylactic shock, angioedema (Quincke's edema), hypersensitivity reactions.
Gastrointestinal disorders
very rarely: peptic ulcer, bleeding, diarrhea.
Skin and subcutaneous tissue disorders
uncommon: local skin reactions such as erythema, eczema, itching and burning;
rarely: photosensitivity reactions, urticaria.
There have been rare reports of more severe reactions, such as bullous or phlyctenulous eczema, which may spread beyond the site of application or become generalized.
Renal and urinary tract disorders
very rare: deterioration of renal function in patients with chronic renal failure.
If any side effects occur, you should stop using the drug and consult a doctor.
Overdose
Overdose is unlikely when the drug is used externally.
In case of overdose, the skin should be thoroughly rinsed under running water.
If the drug is ingested, systemic adverse reactions may develop. In this case, symptomatic treatment and supportive therapy are necessary, as in case of overdose with oral forms.
Interaction with other drugs
When ketoprofen is used externally in a gel dosage form, it is possible to enhance the effect of drugs that cause photosensitivity.
Patients taking coumarin anticoagulants are recommended to regularly monitor the international normalized ratio (INR).
Ketoprofen, like other NSAIDs, may reduce the elimination of methotrexate and increase its toxicity.
Interaction with other drugs and the effect on their elimination are not significant.
special instructions
It is necessary to avoid getting the gel in the eyes, on the skin around the eyes, and mucous membranes. If skin reactions occur, including those that develop during combined use with octocrylene-containing drugs, treatment should be stopped immediately.
Patients suffering from bronchial asthma in combination with chronic rhinitis, chronic sinusitis and/or polyposis of the nose or paranasal sinuses have a higher risk of developing allergic reactions when using aspirin and/or NSAIDs than the rest of the population.
To reduce the risk of developing photosensitivity, it is recommended to protect the skin areas treated with the gel with clothing from exposure to UV radiation throughout the entire treatment period and for another 2 weeks after stopping use. The recommended duration of treatment should not be exceeded due to the increased risk of developing contact dermatitis and photosensitivity reactions over time. You should wash your hands thoroughly after each application of the drug.
The effect of the drug on the ability to drive vehicles and machinery
There is no data on the negative effect of Ketoprofen gel on the ability to drive vehicles and engage in other potentially hazardous activities that require concentration and speed of psychomotor reactions.
Release form
Gel for external use 2.5%.
10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100 g in aluminum tubes with high-density polyethylene caps or in polymer tubes with polyethylene screw caps.
Each tube, along with instructions for medical use, is placed in a cardboard pack.
Storage conditions
At a temperature not higher than 25 °C.
Keep out of the reach of children.
Best before date
3 years.
Do not use after the expiration date stated on the packaging.
Vacation conditions
Available without a prescription.
Analogues of Ketoprofen
Level 4 ATC code matches:
Artrum
Brustan
Ketonal Duo
Nurofen Plus
Nurofen Express
Nurofen Forte
Nurofen Express Lady
Nurofen for children
Nurofen
Ibuprom
Ibuprofen
Advil
MIG 400
Has
Vimovo
Naproxen
Flexen
Nalgesin
Flamax
Novigan
Analogs of Ketoprofen can be considered such drugs as: Artrosilen, Arketal Rompharm, Artrum, Ketonal, Bystrumgel, Valusal, Ketoprofen Organica, Ketoprofen Vramed, Ketoprofen forte, Ketoprofen MV, Ketoprofen-Verte, Ketosprey, Ketoprofen-ESKOM, OKI, Profenil, Oruvel, Fastum , Flamax, Fastum gel, Flexen, Febrofid.
Ketoprofen price, where to buy
The cost of the drug depends, first of all, on its dosage form (gel, suppositories, tablets or injections), as well as the region. Since this drug is quite popular among patients suffering from diseases of the musculoskeletal system, you can buy Ketoprofen in many pharmacies, including on the Internet.
The average price of Ketoprofen gel (50 g tube) is 70 rubles, the price of Ketoprofen tablets is 200-240 rubles.
Ketoprofen ointment is not produced or sold.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Ketoprofen Organica tablets p.p.o.
100 mg 20 pcs. JSC Organika 122 rub. order - Ketoprofen gel 2.5% 50mlVetProm AD
122 RUR order
- Dexketoprofen Organic solution for intravenous and intramuscular injection. 25mg/ml 2ml 10pcs JSC Organika
RUB 321 order
- Ketoprofen Organika capsules 50 mg 20 pcs. JSC Organika
102 RUR order
- Ketoprofen Organic solution for intravenous and intramuscular injection. 50mg/ml ampoules 2ml 10pcs JSC Organika
104 rub. order
Pharmacy Dialogue
- Ketoprofen gel (tube 5% 30g) Sintez (Kurgan) OJSC
RUB 149 order
- Ketoprofen gel (tube 2.5% 50g)Vertex
140 rub. order
- Ketoprofen gel (tube 2.5% 50g)VetProm AD
96 RUR order
- Ketoprofen gel (tube 5% 30g)Vertex
RUB 191 order
- Ketoprofen (amp. 50 mg/ml 2 ml No. 10) Ellara LLC
73 rub. order
show more
Pharmacy24
- Dexketoprofen-Astrapharm 25 mg N10 tablets TOV Astrapharm, Ukraine
55 UAH order
show more
Ketoprofen-vertex 5% 30g gel for external use
pharmachologic effect
NSAID, propionic acid derivative.
It has analgesic, anti-inflammatory and antipyretic effects. The mechanism of action is associated with inhibition of the activity of COX, the main enzyme in the metabolism of arachidonic acid, which is a precursor of prostaglandins, which play a major role in the pathogenesis of inflammation, pain and fever. The pronounced analgesic effect of ketoprofen is due to two mechanisms: peripheral (indirectly, through suppression of prostaglandin synthesis) and central (due to inhibition of prostaglandin synthesis in the central and peripheral nervous system, as well as the effect on the biological activity of other neurotropic substances that play a key role in the release of pain mediators in the spinal cord). brain). In addition, ketoprofen has antibradykinin activity, stabilizes lysosomal membranes, and causes significant inhibition of neutrophil activity in patients with rheumatoid arthritis. Suppresses platelet aggregation.
Composition and release form Ketoprofen-vertex 5% 30g gel for external use
Gel – 1 g:
- Active substance: ketoprofen 50 mg.
- Excipients: carbomer, ethanol 96%, lavender oil, macrogol, diethanolamine to pH 5.0 - 7.5, purified water.
30 g - aluminum tubes (1) - cardboard packs.
Description of the dosage form
| Colorless, transparent gel with the smell of ethyl alcohol. Opalescence is acceptable. |
Directions for use and doses
Set individually depending on the clinical situation.
Used orally, intramuscularly, intravenously (as an infusion), rectally or externally in appropriate dosage forms.
Pharmacokinetics
When taken orally and rectally, ketoprofen is well absorbed from the gastrointestinal tract. Cmax; in plasma when taken orally is achieved after 1-5 hours (depending on the dosage form), with rectal administration - after 45-60 minutes, intramuscular administration - after 20-30 minutes, intravenous administration - after 5 min.
Plasma protein binding is 99%. Due to its pronounced lipophilicity, it quickly penetrates the BBB. Css; in blood plasma and cerebrospinal fluid remains from 2 to 18 hours. Ketoprofen penetrates well into the synovial fluid, where its concentration 4 hours after administration exceeds that in plasma.
Metabolized by binding to glucuronic acid and, to a lesser extent, by hydroxylation.
It is excreted mainly by the kidneys and to a much lesser extent through the intestines. T1/2 of ketoprofen from plasma after oral administration is 1.5-2 hours, after rectal administration - about 2 hours, after intramuscular administration - 1.27 hours, after intravenous administration - 2 hours.
Indications for use Ketoprofen-vertex 5% 30g gel for external use
Articular syndrome (rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, gout); symptomatic treatment of inflammatory and degenerative diseases of the musculoskeletal system (periarthritis, arthrosynovitis, tendonitis, tenosynovitis, bursitis, lumbago), pain in the spine, neuralgia, myalgia. Uncomplicated injuries, in particular sports injuries, sprains, sprains or ruptures of ligaments and tendons, bruises, post-traumatic pain.
As part of combination therapy for inflammatory diseases of the veins, lymphatic vessels, lymph nodes (phlebitis, periphlebitis, lymphangitis, superficial lymphadenitis).
Contraindications
For oral administration: erosive and ulcerative lesions of the gastrointestinal tract in the acute phase, “aspirin triad”, severe dysfunction of the liver and/or kidneys; III trimester of pregnancy; age up to 15 years (for retard tablets); hypersensitivity to ketoprofen and salicylates.
For rectal use: history of proctitis and rectal bleeding.
For external use: weeping dermatoses, eczema, infected abrasions, wounds.
Effect on the body
| Non-steroidal anti-inflammatory drug (NSAID). |
Application of Ketoprofen-vertex 5% 30g gel for external use during pregnancy and breastfeeding
Contraindicated for use in the third trimester of pregnancy. In the first and second trimesters of pregnancy, the use of ketoprofen is possible in cases where the potential benefit to the mother outweighs the potential risk to the fetus.
If it is necessary to use ketoprofen during lactation, it is recommended to stop breastfeeding.
Use in children
Contraindicated under the age of 15 years (for retard tablets).
special instructions
Use with extreme caution in patients with liver and kidney diseases, a history of gastrointestinal diseases, dyspeptic symptoms, and immediately after major surgical interventions. During treatment, systematic monitoring of liver and kidney function is necessary.
Overdose
| The extremely low systemic absorption of the active components of the drug when used externally makes overdose almost impossible. |
Side effects Ketoprofen-vertex 5% 30g gel for external use
From the digestive system: pain in the epigastric region, nausea, vomiting, constipation or diarrhea, anorexia, gastralgia, liver dysfunction; rarely - erosive and ulcerative lesions of the gastrointestinal tract, bleeding and perforation of the gastrointestinal tract.
From the side of the central nervous system: headache, dizziness, tinnitus, drowsiness.
From the urinary system: renal dysfunction.
Allergic reactions: ;skin rash; rarely - bronchospasm.
Local reactions: ; when used in the form of suppositories, irritation of the rectal mucosa and painful bowel movements are possible; when used in gel form - itching, skin rash at the site of application.
Drug interactions
When ketoprofen is used simultaneously with other NSAIDs, the risk of developing erosive and ulcerative lesions of the gastrointestinal tract and bleeding increases; with antihypertensive drugs (including beta-blockers, ACE inhibitors, diuretics) - their effect may be reduced; with thrombolytics - increased risk of bleeding.
When used simultaneously with acetylsalicylic acid, it is possible to reduce the binding of ketoprofen to plasma proteins and increase its plasma clearance; with heparin, ticlopidine - increased risk of bleeding; with lithium preparations - it is possible to increase the concentration of lithium in the blood plasma to toxic levels due to a decrease in its renal excretion.
When used simultaneously with diuretics, the risk of developing renal failure increases due to a decrease in renal blood flow caused by inhibition of prostaglandin synthesis and against the background of hypovolemia.
When used simultaneously with probenecid, the clearance of ketoprofen and its binding to plasma proteins may be reduced; with methotrexate – the side effects of methotrexate may increase.
With the simultaneous use of warfarin, severe, sometimes fatal bleeding may develop.