The last decade can be considered as the beginning of the incretin era in the treatment of type 2 diabetes mellitus (T2DM). The pharmacological implementation of the incretin effect has made it possible to rapidly and quite widely use incretins in global clinical diabetes practice. An important distinctive feature of this innovative class of glucose-lowering drugs is the simultaneous positive effect on two pathogenetic components of T2DM. Incretins not only contribute to an increase in postprandial glucose-dependent insulin secretion by β-cells of the pancreas, but also cause a decrease in the synthesis of the contrainsular hormone glucagon by α-cells and, consequently, the intensity of the process of hepatic gluconeogenesis.
The main incretins of the enteroinsular axis are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Inhibition of dipeptidyl peptidase-4 (DPP-4) by DPP-4 inhibitors (gliptins) allowed the half-life of native GLP-1 to be prolonged from several minutes to several hours (Fig. 1). It should be noted that the therapeutic potential of gliptins is determined by the physiological concentration of GLP-1.
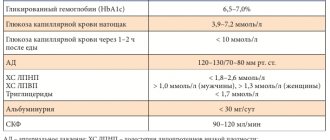
Personalized medicine involves individualizing the prescription of therapy depending on the duration of T2DM, the quality of glycemic control, the risk of hypoglycemia, complications of DM and existing comorbid diseases, age (life expectancy), and anamnestic features [2].
What requirements must modern glucose-lowering drugs meet?
• Undoubtedly and above all, it has a high hypoglycemic potential that persists over a long period of use. • Reducing the level of glycemia based on the maximum physiological effect, which does not cause depletion of the functional activity of pancreatic β-cells. • Reducing glycemic variability and eliminating peaks of postprandial hyperglycemia. • Low incidence of hypoglycemic episodes. • High degree of security. • No decrease in the patient's quality of life. • Patient adherence to therapy, convenient pharmacological form, possibility of producing fixed combinations, minimizing the number of missed doses. • Positive non-glycemic effects. • Lack of weight gain.
According to the consensus and standards adopted in most countries of the world for the management of patients with T2DM, metformin remains the first choice drug in the treatment of T2DM. Its positive role in reducing gluconeogenesis, insulin resistance and the risk of developing cancer has been established. However, for every tenth patient, due to the development of undesirable side effects from the gastrointestinal tract, metformin is unacceptable. Thus, the problem of choosing a second-line drug while taking metformin, a drug for initial therapy in combination with metformin, as well as a first-line drug in case of intolerance to metformin remains. According to the updated ADAEASD consensus (American Diabetes Association-European Association for the Study of Diabetes, 2012), DPP-4 inhibitors are one of the promising therapeutic approaches to the selection of first- and second-line therapy [3].
A review of the market for glucose-lowering drugs indicates that the choice of clinicians is increasingly shifting towards prescribing DPP-4 inhibitors to patients with T2DM. Much of the experience gained from clinical trials (both preclinical and post-marketing) is based on the evaluation and safety of the DPP-4 inhibitor sitagliptin, the first and most studied member of the gliptin class. The drug Januvia (sitagliptin, Merck Sharp & Doum, USA) was approved by the Food and Drug Administration (FDA) in 2006, the European Medicines Agency in 2007. Ongoing and already conducted randomized clinical trials (RCTs) involving sitagliptin constitute a powerful evidence base that allows us to comprehensively outline the effectiveness of its hypoglycemic effect, non-glycemic effect and safety of its use. It should be noted that sitagliptin is used as an interventional or reference comparator drug. The use of sitagliptin in the form of monotherapy, combination with metformin, double and triple combinations of oral hypoglycemic drugs, and combination with insulin has been studied. Post-marketing studies of sitagliptin are ongoing. It should be noted that the clinical effectiveness of the combination of sitagliptin with the first-choice drug metformin served as a prerequisite for the creation of the first fixed combination of a DPP-4 inhibitor and metformin - the drug Janumet (Janumet, Merck Sharp & Doum, USA).
Introduction
According to modern concepts, type 2 diabetes mellitus (DM) is a chronic progressive disease, the prevalence of which continues to increase.
Thus, according to the International Diabetes Federation (IDF), in 2017, in the world there were 425 million people aged 20 to 79 years with diabetes, 5 million deaths were associated with complications of diabetes. According to IDF forecasts, by 2045 the number of people with diabetes is expected to increase to 629 million people, the vast majority of whom will be diagnosed with type 2 diabetes [1]. According to the results of the national NATION study, the actual number of patients with diabetes in our country is at least 9 million people (approximately 6% of the population) [2]. The complex pathophysiology of type 2 diabetes and the tendency to progress require a multifactorial strategy in therapy and continuous monitoring of its effectiveness. Today we know more than 10 pathogenetic factors in the development of type 2 diabetes. Along with progressive β-cell dysfunction and insulin resistance, particular importance is attached to the disruption of the incretin response to meals and increased glucose reabsorption in the renal tubules. One of the innovative classes of drugs that affect the incretin mechanisms regulating carbohydrate metabolism are dipeptidyl peptidase-4 (DPP-4) inhibitors (or gliptins). The drugs block the inactivation of incretin hormones, in particular glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), resulting in increased glucose-dependent insulin secretion and a decrease in postprandial glucagon release (Fig. 1).
Recommendations
- ^ a b c
GRCh38: Ensemble issue 89: ENSG00000197635 — Ensemble, May 2017 - ^ a b c
GRCm38: Ensemble Issue 89: ENSMUSG00000035000 — Ensemble, May 2017 - "Human's Guide to PubMed:". National Center for Biotechnology Information, US National Library of Medicine
. - "Mouse PubMed link:". National Center for Biotechnology Information, US National Library of Medicine
. - Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto S. (July 1993). "Direct association of adenosine deaminase with T cell activation antigen, CD26". The science
.
261
(5120):466–9. Bibcode:1993Science… 261..466K. Doi:10.1126/science.8101391. PMID 8101391. - Khopsu-Khavou V.K., Glenner G.G. (1966). "A new dipeptide naphthylamidase that hydrolyzes glycyl-prolyl-beta-naphthylamide." Histochemie.
Histochemistry. Histochimie .
7
(3): 197–201. Doi:10.1007/bf00577838. PMID 5959122. S2CID 9674831. - Vanhoof J, Goossens F, De Meester I, Hendricks D, Sharpe S (June 1995). "Proline motifs in peptides and their biological processing". FASEB Magazine
.
9
(9): 736–44. Doi:10.1096/fasebj.9.9.7601338. PMID 7601338. S2CID 37551773. - ^ a b
Mentlein R (November 1999).
"Dipeptidyl peptidase IV (CD26) - role in inactivation of regulatory peptides." Regulatory peptides
.
85
(1):9–24. Doi:10.1016/S0167-0115 (99) 00089-0. PMID 10588446. S2CID 22354304. - Chen X (2006). "Biochemical properties of recombinant prolyl dipeptidases DPP-IV and DPP8". Dipeptidylaminopeptidases
.
Advances in experimental medicine and biology. 575
. pp. 27–32. Doi:10.1007/0-387-32824-6_3. ISBN 978-0-387-29058-4. PMID 16700505. - Barnett A. (November 2006). "DPP-4 inhibitors and their potential role in the treatment of type 2 diabetes." International Journal of Clinical Practice
.
60
(11): 1454–70. Doi:10.1111/j.1742-1241.2006.01178.x. PMID 17073841. S2CID 2645092. - Pro B, Dang NH (October 2004). "CD26/dipeptidyl peptidase IV and its role in cancer development". Histology and histopathology
.
19
(4): 1345–51. Doi:10.14670/HH-19.1345. PMID 15375776. - Mazur K., Schwarz F., Entschladen F., Niggemann B., Zaenker K.S. (December 2006). "DPPIV Inhibitors Extend GLP-2-Mediated Tumor Effects on Colon Cancer Cells." Regulatory peptides
.
137
(3):147–55. doi:10.1016/j.regpep.2006.07.003. PMID 16908079. S2CID 2857735. - Wesley WF, McGroarty M, Homoyouni A (February 2005). "Dipeptidyl peptidase inhibits the malignant phenotype of prostate cancer cells by blocking the basic fibroblast growth factor signaling pathway." Cancer Research
.
65
(4): 1325–34. Doi:10.1158/0008-5472.CAN-04-1852. PMID 15735018. - Busek P., Malik R., Sedo A. (March 2004). "Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer". International Journal of Biochemistry and Cell Biology
.
36
(3): 408–21. Doi:10.1016/S1357-2725(03)00262-0. PMID 14687920. - Kaji K., Yoshiji H., Ikenaka Y., Noguchi R., Aihara Y., Duhara A., Moriya K., Kawaratani H., Shirai Y, Yoshii J., Yanase K., Kitade M., Namisaki T. , Fukui H (March 2014). "Dipeptidyl peptidase-4 inhibitor attenuates liver fibrosis through suppression of activated hepatic stellate cells in rats." Journal of Gastroenterology
.
49
(3):481–91. Doi:10.1007/s00535-013-0783-4. PMID 23475323. S2CID 2726091. - Min HS, Kim J, Lee MH, Song HK, Kang YS, Lee MJ, Lee J, Kim HW, Cha JJ, Chang YY, Hyun Y, Han J, Cha D .R. "Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction." Laboratory research;
Journal of Engineering Methods and Pathology .
94
(6):598–607. Doi:10.1038/labinvest.2014.50. PMID 24687121. S2CID 23745972. - Le Havre, PA, Abe M, Urasaki Y, Ohnuma K, Morimoto S, Dang NH. (January 2008). "The role of CD26/dipeptidyl peptidase IV in cancer". Frontiers in Biological Sciences
.
13
(13): 1634–45. doi:10.2741/2787. PMID 17981655. - Rosenstock J, Zinman B (April 2007). "Dipeptidyl peptidase-4 inhibitors and the treatment of type 2 diabetes mellitus." Current Opinion in Endocrinology, Diabetes and Obesity
.
14
(2):98–107. Doi:10.1097/MED.0b013e3280a02f65. PMID 17940427. S2CID 25482131. - Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL (March 2013 G.) ). "Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus - EMC." Nature
.
ScienceNews. 495
(7440):251–4.
Bibcode:2013Natura.495..251 rub. doi:10.1038/nature12005. PMC 7095326. PMID 23486063. Add a resume - ScienceNews
.
Incretin effect
The basis of the incretin effect (“incretin” - INtestinal seCRETion INsulin, “gut-mediated” insulin secretion) is a more powerful stimulation of insulin secretion in response to the intake of glucose from food compared to parenteral administration of glucose in an equivalent dose, which is possible due to the production of incretin hormones ( GLP-1 and GIP) in intestinal endocrine K- and L-cells and their effects on β-cells. It is known that due to the incretin effect, healthy people produce up to 50–70% of the daily dose of insulin. After eating, the concentration of GLP-1 in the blood increases 2–3 times [3]. In addition to glucose-dependent insulin secretion, the biological effects of GLP-1 also include stimulation of insulin biosynthesis, inhibition of glucagon secretion, slowing gastric emptying, and the onset of a feeling of satiety [4]. A comparison of the effect of infusion of GLP-1 and GIP in people with type 2 diabetes demonstrated that the initial early phase of insulin secretion was restored during the administration of GIP, and the late phase of insulin secretion (from 20 to 120 min) was maintained in response to GLP-1 administration. . Thus, the strategy of activating the incretin response has become one of the most relevant in the treatment of type 2 diabetes [3]. And one of the ways of such activation is inhibition of the enzyme DPP-4, which destroys up to 50% of GLP-1 in the first 1-2 minutes after entering the bloodstream. The emergence of DPP-4 inhibitors made it possible to maintain the incretin response and adequate postprandial regulation of carbohydrate metabolism in patients with type 2 diabetes.
GLP-1 analogues in the treatment of type 2 diabetes mellitus
Currently, there are two drugs of GLP-1 analogues on the Russian market - Bayetta (exenatide) and Victoza (liraglutide). These drugs are synthetic analogues of human GLP-1, but the duration of action is much longer. They have absolutely all the effects of the human hormone that I mentioned above. This is definitely a plus. Another benefit is a decrease in body weight by an average of 4 kg over 6-12 months. and a decrease in glycated hemoglobin by an average of 0.8-1.8%. You can find out what glycated hemoglobin is and why you need to monitor it by reading the article “Glycated hemoglobin: how to test?”.
The disadvantages include:
- Only subcutaneous administration, i.e. no tablet forms.
- The concentration of GLP-1 can increase 5 times, which increases the risk of hypoglycemic conditions.
- The effects of only GLP-1 are increased; the drug does not affect GIP.
- In 30-40%, side effects may occur in the form of nausea and vomiting, but they are transient.
Byeta is available in disposable syringe pens (similar to insulin syringe pens) in a dose of 250 mcg per 1 mg. Pens come in 1.2 and 2.4 ml volumes. One pack contains one pen. Treatment of diabetes mellitus begins with a dose of 5 mcg 2 times a day for 1 month to improve tolerability, and then, if necessary, the dose is increased to 10 mcg 2 times a day. Further increase in dose does not enhance the effect of the drug, but increases the number of side effects.
The Byeta injection is given an hour before breakfast and dinner; it should not be given after meals. If an injection is missed, the next one is given at the appointed time according to the schedule. The injection is given subcutaneously in the thigh, abdomen or shoulder. It cannot be administered intramuscularly or intravenously.
The drug should be stored in a dark, cool place, i.e. on the refrigerator door, do not allow it to freeze. The syringe pen should be stored in the refrigerator each time after an injection. After 30 days, the syringe pen with Byeta is thrown away, even if the drug remains in it, since after this time the drug is partially destroyed and does not have the desired effect. You cannot store the drug you are using with a needle attached, i.e., after each use, the needle must be unscrewed and thrown away, and a new one must be put on before a new injection.
Byeta can also be combined with other glucose-lowering drugs. If the drug is combined with sulfonylurea drugs (Maninil, Diabeton, etc.), then their dose should be reduced to avoid the development of hypoglycemia. There is a separate article about hypoglycemia, so I recommend following the link and studying if you have not already done so. If Byeta is used in conjunction with metformin, the dose of metformin does not change, since hypoglycemia in this case is unlikely.
Victoza is also available in syringe pens in a dose of 6 mg per 1 ml. The volume of the syringe pen is 3 ml. Sold in 1, 2 or 3 syringe pens per package. Storage and use of the syringe pen is similar to Bayeta. Treatment of diabetes mellitus with Victoza is carried out once a day at the same time, which the patient can choose, regardless of food intake. The drug is injected subcutaneously into the thigh, abdomen or shoulder. It also cannot be used for intramuscular and intravenous administration.
The initial dose of Victoza is 0.6 mg per day. After 1 week, you can gradually increase the dose to 1.2 mg. The maximum dose is 1.8 mg, which can be started 1 week after increasing the dose to 1.2 mg. It is not recommended to administer the drug above this dose. By analogy with Bayeta, Victoza can be used with other glucose-lowering drugs.
And now about the most important thing - the price and availability of both drugs. This group of drugs is not included in either the federal or regional list of preferential drugs for the treatment of patients with diabetes. Therefore, you will have to buy these drugs with your own money. To be honest, these drugs are not cheap. The price depends on the dose of the medicine administered and the packaging. For example, Byeta 1.2 mg contains 60 doses of the drug. This amount is enough for 1 month. provided that the prescribed daily dose is 5 mcg. In this case, the drug will cost you an average of 4,600 rubles per month. If it is Victoza, then with a minimum daily dose of 6 mg the drug will cost 3,400 rubles per month.
Dipeptidyl peptidase type 4
DPP-4 was first isolated as a membrane glycoprotein of CD-26 T cells, acting as a binding protein or ligand for molecules. Today it is known that DPP-4 is an integral protein that is not only capable of being expressed in all cells of the body, but also circulates in the bloodstream in free form as an enzyme with catalytic activity [5]. In addition to incretin hormones, physiological substrates for DPP-4 are various cytokines, growth factors and neuropeptides, which have a regulatory effect on the functioning of all organs and systems of the body, including the neuroendocrine system. DPP-4 is present in large quantities in vascular endothelial cells, which makes all peptide molecules circulating through the intestines, liver, lungs and kidneys accessible to it [3, 6]. It is therefore hypothesized that DPP-4 may additionally influence metabolic control through its proteolytic action on other regulatory peptides and even influence insulin sensitivity, potentially mediated by its non-enzymatic interactions with other membrane proteins. Today, DPP-4 is considered both as a local mediator of inflammation and insulin resistance in adipose and liver tissue, and as a participant in the paracrine system, including the local production of GLP-1 in the pancreatic islets. Obesity has been shown to be associated with increased levels of soluble DPP-4, which is overexpressed in hepatocytes and adipocytes. An increase in DPP-4 in the bloodstream leads, in turn, to adipocyte insulin resistance, which is presumably caused by caveolin-1 (Cav-1), produced on the membrane of adipocytes themselves and in adipose tissue macrophages [5] (Fig. 2).
Interest in the DPP-4 enzyme has further intensified with the discovery and approval of highly selective DPP-4 iAs, agents that selectively inactivate DPP-4 and enhance the predominantly incretin effect for the treatment of type 2 diabetes [7].
DPP-4 inhibitors
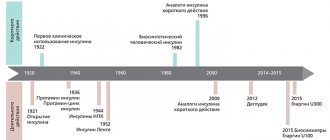
Sitagliptin became the first agent to be approved for the treatment of type 2 diabetes back in 2006. To date, more than 10 drugs of the DPP-4 inhibitor group have entered clinical practice around the world. Until recently, 6 medicinal names were registered in Russia: sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin, gozogliptin. In 2022, a new drug appeared - evogliptin. All drugs are in tablet form and are prescribed at a frequency of 1–2 times a day. DPP-4 inhibitors demonstrate inhibition of enzyme activity within 5 minutes after administration [3].
According to domestic and foreign data, DPP-4 inhibitors still remain reserve/second-choice drugs when choosing initial monotherapy for type 2 diabetes [2, 8]. Most often prescribed in combination with lifestyle modification and in combination with other classes (metformin, sulfonylureas (SMUs), thiazolidinediones, sodium-glucose cotransporter type 2 inhibitors and/or basal insulin). But in case of intolerance or contraindications to taking metformin (for example, chronic kidney disease), DPP-4 inhibitors can be an excellent option, both in monotherapy and in combinations with drugs of other classes.
The hypoglycemic effectiveness of DPP-4 is expressed in a decrease in the level of glycated hemoglobin (HbA1c) by 0.5-1%, which is inferior to PSMs, which reduce HbA1c by 1-2%. In addition, DPP-4 inhibitors are less affordable. And at first glance it may seem that PSM is “more powerful” than iDPP-4 and is more economical. Indeed, in all countries of the world, PSMs occupy one of the leading positions in terms of frequency of prescription. But is this justified? After all, 15 years of experience in using drugs from the DPP-4 group confirms their standard safety and satisfactory glucose-lowering ability. There is evidence of marked differences between DPP-4 i and PSM in relation to the development of cardiovascular events, microvascular complications and expected long-term prognosis. According to the Swedish National Registry, during DPP-4 therapy there is a decrease in the incidence of cardiovascular events and severe hypoglycemia [9]. According to numerous studies, DPP-4 i, unlike PSM, have a neutral effect on body weight and the risk of hypoglycemia (which is explained by glucose-dependent stimulation of insulin secretion), unless there is concomitant treatment with insulin [10]. However, several clinical trials have shown a decrease in HbA1c levels without an increase in the risk of hypoglycemia when adding a DPP-4 inhibitor to basal insulin therapy [11].
According to a meta-analysis by K. Chen et al. With the inclusion of results from long-term clinical trials, it was found that the effect of DPP-4 inhibitors on HbA1c levels in patients with type 2 diabetes may weaken during the 2nd year of treatment. However, a direct comparison of “glycemic longevity” with PSM reliably showed that in the period from the 24th to the 104th week of therapy against the background of DPP-4 inhibitors, a less pronounced increase in HbA1c levels was observed - on average by 0.16% (p < 0.001). The data obtained suggest a more durable glycemic response to DPP-4 inhibitors compared to PSM when therapy lasts more than 2 years [12].
One of the main advantages of DPP-4 inhibitors is the possibility of prescribing even to patients with end-stage renal failure, including those on dialysis [2, 3]. DPP-4 inhibitors have occupied a key place in the treatment strategy for type 2 diabetes in elderly patients. Good tolerability, cardiovascular safety, low risk of hypoglycemia (even in combination with basal insulin) and the possibility of administration in severe renal failure have made the DPP-4 group more preferable for older people than PSM [13–16]. It is worth noting that DPP-4 inhibitors are included in combination drugs such as gliptin + metformin, gliptin + pioglitazone and gliptin + sodium glucose cotransporter type 2 inhibitor, which significantly increases adherence to therapy, especially in elderly patients with polypharmacy. European scientists note that in real clinical practice, the prescription of a combination of insulin and PSM to elderly patients continues to prevail, while the strategic goal in the treatment of this category of people is to maintain target blood glucose levels while minimizing the risk of hypoglycemia and thereby maintaining a high quality of life. According to their data, the choice in favor of DPP-4 inhibitors over a 4-year follow-up period not only improved glycemic control in elderly patients, but also made it possible to achieve this goal safely and with improved quality of life (as assessed by the patients themselves and their relatives) [17 -19].
Thus, the advantages of DPP-4 iDPP-4 over PSM are undeniable, such as no weight gain, low risk of hypoglycemia, no need for dose titration, acceptability for patients with severe renal failure, longer retention of the hypoglycemic effect and preferential safety when administered to elderly patients.
Of the incretin drugs, DPP-4 inhibitors are less effective in reducing HbA1c and normalizing body weight than GLP-1 receptor agonists, but their undeniable advantages even over GLP-1 agonists remain the oral form of administration and affordability.
Drugs based on the action of incretins and intended for the treatment of type 2 diabetes mellitus (T2DM) appeared in clinical practice in 2005. The central place among incretins is occupied by glucagon-like peptide-1 (GLP-1), an intestinal hormone that plays an important role in maintaining normal carbohydrate metabolism. The beneficial effects of GLP-1 include improvements in β-cell function, including glucose-dependent insulin secretion and suppression of glucagon production, as well as a number of extrapancreatic properties such as inhibition of gastric emptying and appetite suppression [1].
However, while in the bloodstream, GLP-1 does not exert its effects for long due to its rapid destruction and elimination from the body. The enzyme that is responsible for the degradation of GLP-1 and the loss of its insulinotropic effect is the serine protease dipeptidyl peptidase-4 (DPP-4). Understanding the role of DPP-4 in the metabolism of GLP-1 gave rise to the creation of inhibitors of this enzyme in order to increase the duration of action of endogenous GLP-1 [2]. The results of preclinical studies demonstrating the preservation of GLP-1 concentrations under the influence of DPP-4 inhibitors [3] were later confirmed among patients with T2DM, in whom taking DPP-4 inhibitors reduced glycemic levels [4].
Representatives of the class of DPP-4 inhibitors (gliptins) are firmly entrenched in the market of glucose-lowering drugs. These include sitagliptin, vildagliptin, saxagliptin, alogliptin (registered in Japan), linagliptin (registered in the USA and Europe). This article presents a comparative description of three DPP-4 inhibitors registered in the Russian Federation: sitagliptin (Januvia), vildagliptin (Galvus), saxagliptin (Ongliza).
Chemical formula
All DPP-4 inhibitors bind reversibly to the active site of the enzyme. Sitagliptin forms a non-covalent bond with DPP-4, while vildagliptin and saxagliptin bind to the active site of the enzyme via a covalent bond (Fig. 1).
Figure 1. Chemical structure of DPP-4 inhibitors. The covalent bond leads to the formation of a stable enzyme-inhibitor complex, characterized by a low dissociation rate, which allows the inhibitor to remain active even after the drug is eliminated [5–7]. This explains why vildagliptin and saxagliptin take longer to exert their effects than their half-life would suggest.
Pharmacodynamics of DPP-4 inhibitors
In therapeutic doses, all gliptins lead to a long-term decrease in enzyme activity throughout the day. The degree of DPP-4 inhibition reaches more than 90% 15 minutes after taking the drug and remains at the level of 70-90% over the next 24 hours [8-10], which leads to a significant increase in the concentration of GLP-1 (1.5-4 times). Data were obtained for sitagliptin at a daily dose of 100 mg, saxagliptin 5 mg and vildagliptin 100 mg (50 mg 2 times a day).
As is known, DPP-4 is a member of the dipeptidyl peptidase family, two of which (DPP-8 and DPP-9), according to some studies [11, 12], are responsible for T-cell activation and proliferation. In in vitro studies, sitagliptin demonstrated the greatest selectivity for DPP-4 [13, 14], while vilda- and saxagliptin had moderate selectivity [15, 16]. The high selectivity of sitagliptin suggests minimization of possible side effects, however, given the intracellular localization of DPP-8 and -9, the significance of high selectivity in vivo remains unclear. Gliptins are not substrates, inducers or inhibitors of CYP450 enzymes, with the exception of saxagliptin, the active metabolite of which is formed by the cytochrome P3A4/5 enzyme [13, 17, 18].
Pharmacokinetics of DPP-4 inhibitors
DPP-4 inhibitors are available in oral form. The maximum concentration of the drugs is achieved in less than 4 hours. Sitagliptin and vildagliptin have high bioavailability (~85-87%), slightly lower for saxagliptin (~67%) [19-21]. DPP-4 inhibitors reversibly and to a small extent bind to plasma proteins (38% for sitagliptin, 10% for vildagliptin, insignificant for saxagliptin), which determines the predominantly renal route of their elimination. Sitagliptin has its main effect in unchanged form, vildagliptin is metabolized to an inactive metabolite, saxagliptin to an active one [17-19, 22]. In this regard, when prescribing saxagliptin together with CYP3A4/5 inhibitors, such as ketoconazole or clarithromycin, it is recommended to reduce the dose of saxagliptin to 2.5 mg per day [23]. Gliptins are excreted mainly in the urine, which implies the need to reduce the dose of drugs as GFR decreases.
The main parameters of metabolism of DPP-4 inhibitors are presented in table. 1.
Use in renal and liver failure
Considering that DPP-4 inhibitors are excreted primarily through the kidneys, the development of renal failure requires dose adjustment of these drugs. DPP-4 inhibitors are approved for use in a therapeutic dose with a moderate decrease in GFR (50-80 ml/min/1.73 m2), with GFR below 50 ml/min/1.73 m2, the dose of drugs should be reduced by 50% or more [17, 18, 24].
Most studies have shown that dose adjustment of sitagliptin and vildagliptin is not required when liver failure develops. However, caution is required when prescribing drugs of this group in individuals with an increase in liver transaminases of more than three norms and quarterly monitoring of enzymes during the first year of using DPP-4 inhibitors.
Sugar-lowering effect in monotherapy
The glucose-lowering activity of DPP-4 inhibitors was studied in large, randomized, placebo-controlled clinical trials, which demonstrated a statistically significant decrease in HbA1c with gliptins.
In a 12-week study evaluating the glucose-lowering effect of sitagliptin
, use of the drug at a dose of 100 mg 1 time per day led to a decrease in HbA1c by an average of 0.6% compared to placebo (p <0.001) [25, 26]. Moreover, the observed difference was greater, the higher the initial HbA1c value was: with an initial HbA1c level of less than 7%, its decrease was -0.4%; in patients with an initial HbA1c level of 7-8.5%, the indicator decreased by -0.6% ; finally, with HbA1c 8.5-10%, its decrease reached –0.8%.
In a 24-week study, different dosages of vildagliptin
(50 mg once daily/50 mg twice daily/100 mg once daily) were prescribed to patients who had not previously received therapy [27]. The decrease in HbA1c was 0.7-0.9±0.1% during treatment with vildagliptin compared to placebo (p<0.001). Prescribing vildagliptin to patients with higher HbA1c values was also associated with a more pronounced reduction in this indicator.
Use of saxagliptin
among patients who had not previously received PSSP, was associated with a decrease in HbA1c by 0.7-0.9% depending on the dosage (2.5/5/10//20/40 mg), with the greatest effectiveness being obtained at a dose of 5 mg 1 time per day. In the placebo group, the reduction in HbA1c was 0.27% [28].
Sugar-lowering effect in combination with other PSSPs
Addition of 100 mg sitagliptin
to metformin therapy in patients with poor glycemic control (HbA1c ≥7% and ≤10%) resulted in a significant reduction in HbA1c (approximately -0.7% compared with placebo, p < 0.001) [29]. The addition of sitagliptin to glimepiride and/or metformin in patients with HbA1c≥7.5 and ≤10.5% resulted in a decrease in HbA1c by an average of 0.74% compared to placebo (p<0.001): in the glimepiride+metformin subgroup, the addition of sitagliptin led to a decrease in HbA1c by 0.89%, in the subgroup taking only glimepiride - by 0.57% [30].
Vildagliptin combination
with metformin in patients with inadequate glycemic control significantly led to a decrease in HbA1c levels: in patients who had previously taken metformin, the addition of vildagliptin at a dose of 50 mg per day caused a decrease in HbA1c by 0.7%, at a dose of 50 mg 2 times a day - by 1 .1% [31]. In a study using the combination of vildagliptin with glimepiride, HbA1c decreased from baseline 8.3 to 6.8% 12 months after the start of therapy [32].
Vildagliptin and sitagliptin are approved for use in combination with insulin. At a dose of 50 mg twice daily, the addition of vildagliptin to initial NPH insulin therapy significantly reduced HbA1c by 0.5% in the general population; among patients over 65 years of age, the reduction in HbA1c reached –0.7% compared to placebo (–0.1%) [33]. There were no cases of severe hypoglycemia recorded in the study.
There are few comparative studies of drugs from the group of DPP-4 inhibitors, one of them was carried out in relation to a combination of sita- and saxagliptin
with metformin among patients with unsatisfactory glycemic control (HbA1c 6.5-10% while taking 1.5-3 g metformin per day) [34]: the addition of 5 mg saxagliptin and 100 mg sitagliptin led to a decrease in HbA1c by an average of 0. 5 and 0.6% respectively.
A direct comparison of the two most studied members of the DPP-4 inhibitor class, sitagliptin and vildagliptin, was conducted in patients with T2DM who were poorly controlled on metformin. The addition of vildagliptin or sitagliptin to therapy for 3 months of therapy provided an average reduction in HbA1c by -0.9% compared to placebo (R. Marfella R et al.) [35]. In addition, similarity in the dynamics of the main glycemic parameters (HbA1c, fasting and postprandial plasma glucose) was demonstrated during treatment with both drugs, but revealed differences in the amplitude of glycemic fluctuations, GLP-1 activity and the degree of glucagon suppression. Vildagliptin promoted a more pronounced increase in GLP-1 levels between meals, more strongly suppressed glucagon secretion, and significantly reduced the amplitude of glycemic fluctuations [36] (Fig. 2).
Figure 2. Plasma GLP-1 levels while taking vildagliptin and sitagliptin for 3 months. The smaller effect of sitagliptin on the concentrations of GLP-1 and glucagon between meals is probably due to the high rate of its dissociation from the active site of the enzyme (10 s as opposed to 55 min for vildagliptin). At the same time, the short duration of the study and the small group of patients included in the study do not allow us to draw definitive conclusions about whether these differences can play a significant role in terms of clinical significance.
Effect on β- and α-cell function
In clinical studies of all presented DPP-4 inhibitors, improvements in HOMA-β and insulin to proinsulin ratios, which indirectly reflect β-cell function, were noted [37]. Administration of vildagliptin to patients with impaired glucose tolerance (IGT) also improved indices of islet function [38]; however, there are no indications for the use of vildagliptin in patients with IGT to prevent the development of T2DM.
In addition to their effects on β-cells, DPP-4 inhibitors have demonstrated the ability to improve glucose sensitivity and α-cells [37]. Patients with type 2 diabetes exhibit α-cell dysfunction, leading to increased glucagon secretion and a corresponding increase in postprandial hepatic glucose production [39]. Increasing the concentration of incretins by administering sitagliptin, vildagliptin and saxagliptin led to a decrease in α-cell resistance to glucose and a decrease in high glucagon concentrations.
Side effects
In general, the group of DPP-4 inhibitors has a favorable tolerability and safety profile compared to other classes of PSAPs [40]. Unlike sulfonylureas and thiazolidinediones, DPP-4 inhibitors do not cause weight gain, which is of particular importance in the context of a large number of overweight and obese patients. Taking gliptins is not accompanied by the development of edema. Metformin, GLP-1 agonists, and α-glucosidase inhibitors cause gastrointestinal distress, while DPP-4 inhibitors are well tolerated. One of the main advantages of the new class of PSSP is the absence of the risk of hypoglycemia. This property comes from the very principle of action of incretins - a glucose-dependent mechanism of stimulating insulin secretion by beta cells and suppressing glucagon secretion by alpha cells. The low risk of hypoglycemia is an important feature of DPP-4 inhibitors, which gives this class an advantage over sulfonylureas.
The DPP-4 enzyme is involved in the regulation of some inflammatory mediators, so theoretically one can expect a slight increase in the risk of upper respiratory tract infections, which has not yet been confirmed in real clinical practice. As discussed earlier, liver enzymes may increase during vildagliptin therapy, so their monitoring is necessary during the 1st year of treatment.
Since the introduction of sitagliptin into practice, reports of cases of acute pancreatitis in patients taking it began to appear. In July 2011, the journal Gastroenterology published an article assessing the risk of developing pancreatitis, as well as pancreatic and thyroid cancer in patients receiving incretin-stimulating therapy [41]. The authors analyzed the safety of the DPP-4 inhibitor sitagliptin and the GLP-1 mimetic exenatide based on post-marketing adverse event data that were sent to the FDA for evaluation. Traditional PSSPs (rosiglitazone, nateglinide, repaglinide, glipizide) were used as a control group. As a result of the analysis, the authors concluded that the use of exenatide and sitagliptin increases the risk of developing pancreatitis and pancreatic cancer; use of exenatide (but not sitagliptin) increases the risk of developing thyroid cancer. The correctness of the methodology of this study remains controversial, since the FDA adverse event database cannot be used for high-quality calculations and analysis. The correctness of the choice of drugs for the control group is also questionable. A currently available analysis of data from 19 controlled studies involving 10,246 patients with T2DM treated with sitagliptin for 2 years did not reveal an increased risk of pancreatitis [42]. Further study of the potential association between incretin-stimulating therapy in patients with T2DM and the development of pancreatitis or cancer of any location is required.
Combination drugs
DPP-4 inhibitors have proven glycemic control activity and do not cause severe side effects or deplete pancreatic reserves. Such properties of the new class of PSSP help to use it most effectively in patients with newly diagnosed T2DM. The traditional starting therapy drug for patients with T2DM is metformin. The idea of combining two drugs with the safest action profile was embodied in the creation of combined forms of vildagliptin/sitagliptin with metformin (GalusMet and YanuMet). Taking into account the pharmacodynamics of metformin, both drugs are prescribed 2 times a day, which allows maintaining stable inhibition of DPP-4 throughout the day. The combination of a DPP-4 inhibitor and metformin provides an additional glucose-lowering effect compared to monotherapy with each drug and does not increase the side effects of metformin on the gastrointestinal tract. This combination is aimed at achieving target values of glycated hemoglobin in a larger number of patients with T2DM and, possibly, can delay the prescription of sulfonylurea derivatives and the start of insulin therapy.
Conclusion
Since their introduction in 2005, DPP-4 inhibitors have managed to take a strong place among drugs for the treatment of T2DM. The low risk of hypoglycemia, the absence of an effect on body weight and the absence of side effects from the gastrointestinal tract distinguish this class from other PSSPs. Differences among gliptins relate to their chemical structure, ability to inhibit DPP-4, duration of action, metabolism and elimination. At the same time, the data available today indicate their identity in terms of hypoglycemic activity, safety and tolerability. Gliptins can be prescribed to patients with newly diagnosed T2DM, with poor tolerance or contraindications to the administration of biguanides, as well as in combination with other oral hypoglycemic drugs. Vildagliptin and sitagliptin have indications for use with insulin drugs, which opens up new possibilities for combination therapy in patients with a long course of the disease. A distinctive feature of their use is a fixed dosage, no need for titration, as well as a low risk of hypoglycemic episodes and no risk of developing severe hypoglycemic episodes. Research on the effects of DPP-4 inhibitors on preventing loss of β-cell function is encouraging. However, long-term follow-up is needed to confirm these findings and their relevance for clinical practice.
Cardiovascular safety of DPP-4 inhibitors
Data regarding cardiovascular safety were obtained for each of the gliptins: vildagliptin - in independent meta-analyses taking into account patients with congestive heart failure and moderate/severe renal failure, saxagliptin - in the randomized, placebo-controlled SAVOR-TIMI trial among patients with confirmed atherosclerotic cardiovascular disease. vascular disease and/or the presence of risk factors, alogliptin in the EXAMINE study among patients with recent myocardial infarction or hospitalization for unstable angina, sitagliptin in the TECOS study, linagliptin in the CARMELINA (placebo-controlled) study among patients at high cardiovascular risk ) and CAROLINA (compared with glimepiride) [20–26]. Only saxagliptin and alogliptin showed a slight increase in the incidence of hospitalization for cardiovascular failure, so DPP-4 inhibitors should be prescribed with caution in patients with chronic heart failure.
To date, there is no doubt about the safety of DPP-4 therapy in relation to cardiovascular events, as confirmed in the meta-analysis by Dan Liu et al. The meta-analysis included 157,478 patients with type 2 diabetes, of which more than 76 thousand received DPP-4 inhibitors, 81 thousand people were included in the control group. It was shown that during a mean follow-up period of 52 to 152 weeks. The primary endpoint (CV death/non-fatal myocardial infarction/non-fatal stroke) was not significantly different in patients with type 2 diabetes treated with DPP-4 inhibitors compared with controls. Also, mortality from all causes, hospitalization for cardiovascular complications and heart failure did not differ in both groups (Fig. 3) [27].
At present, there is reason to talk not only about the safety of DPP-4 inhibitors, but also about their protective role in relation to the cardiovascular system. Recently, de Oliveira et al. demonstrated improved endothelial function with DPP-4 inhibition in mice exposed to chronic stimulation of the β-adrenergic sympathetic system. Researchers have shown that activation of β-adrenergic receptors can increase the activity of DPP-4, which led to the expression of cytokines in endothelial cells and vascular dysfunction, which was completely reversible with the administration of DPP-4 [28]. In addition, rather contradictory data have emerged demonstrating the effect of DPP-4 inhibitors not only on endothelial function, but also on coronary blood flow and left ventricular diastolic function. In a review by N. Kensuke et al. the authors concluded that a possible positive effect of DPP-4 inhibitors on the cardiovascular system can be expected in patients with initially high HbA1c levels, since the positive effect was demonstrated mainly in studies conducted in patients with decompensated carbohydrate metabolism [29].
further reading
- Ansorge, S., Bühling, F., Kahne, T., Lendekel, U., Reinhold, D., Tager, M., and Wrenger, S. (1997). "CD26/dipeptidyl peptidase IV in the regulation of lymphocyte growth". Advances in experimental medicine and biology
.
421
: 127–40. Doi:10.1007/978-1-4757-9613-1_17. ISBN 978-1-4757-9615-5. PMID 9330689. - Reinhold, D., Koehne, T., Steinbrecher, A., Wrenger, S., Neubert, C., Ansorge, S., Brocke, S. (2003). "The role of dipeptidyl peptidase IV (DP IV) enzymatic activity in T cell activation and autoimmunity". Biological Chemistry
.
383
(7–8):1133–8. Doi:10.1515/BC.2002.123. PMID 12437097. S2CID 30027839. - Sato K., Dang N.H. (March 2003). “CD26: a new target for the treatment of lymphoid T cell malignancies? (Review)". International Journal of Oncology
.
22
(3): 481–97. Doi:10.3892 / ijo.22.3.481 (inactive 10/11/2020). PMID 12579300.CS1 maint: DOI inactive as of October 2020 (link) - de Mister I, Lambeir AM, Proost P, Scharpé S (2003). Dipeptidyl peptidase IV substrates.
Update on in vitro peptide hydrolysis by human DPPIV .
Advances in experimental medicine and biology. 524
. pp. 3–17. Doi:10.1007/0-306-47920-6_1. ISBN 0-306-47717-3. PMID 12675218. - Koch S, Antonsen D, Skovbjerg H, Sjöström H (2003). "On the role of dipeptidyl peptidase IV in the digestion of an immunodominant epitope in celiac disease." Dipeptidylaminopeptidases in health and disease
.
Advances in experimental medicine and biology. 524
. pp. 181–7. Doi:10.1007/0-306-47920-6_22. ISBN 0-306-47717-3. PMID 12675238. - Pro B, Dang NH (October 2004). "CD26/dipeptidyl peptidase IV and its role in cancer development". Histology and histopathology
.
19
(4): 1345–51. Doi:10.14670/HH-19.1345. PMID 15375776.
Possible side effects of DPP-4 inhibitors
One of the side effects that has been associated with DPP-4 inhibitors is the development of acute pancreatitis. However, a cause-and-effect relationship has not been established to date and there is no reliable data confirming an increased risk of developing pancreatitis when using incretin-type drugs [30–32]. However, if acute pancreatitis is confirmed, DPP-4 therapy should be discontinued.
In response to speculation about the possible development of acute renal failure and respiratory tract infections, JM Gamble et al. analyzed the UK Clinical Trials Database between 2007 and 2016 and conclusively demonstrated that initiation of DPP-4 inhibitors was not associated with an increased risk of these complications compared with other drug classes (DSM, metformin, thiazolidinediones and insulin) [33] .
Today, the increased risks of developing inflammatory bowel diseases while taking DPP-4 inhibitors are actively discussed, but there is no convincing data yet [34].
New DPP-4 inhibitor evogliptin
In real clinical practice, according to the DM Register, the most common combination remains therapy with metformin and PSM. The widespread use of PSM is explained by their low price category. However, currently there is a drug from the DPP-4 group, evogliptin, which has an optimal cost, which makes it possible to consider it as an alternative to PSM.
Evogliptin is the newest drug in the gliptin class, sharing all the unique pharmacokinetic properties of DPP-4 inhibitors. An international randomized, double-blind study, EVOCOMBI, was conducted, according to which evogliptin showed safety and efficacy comparable to those of sitagliptin. Data were obtained from Korean and Russian populations of patients who did not achieve the target glycemic level on therapy with metformin alone at a dose of 1000–2000 mg/day [35]. The study involved 281 people, of whom 142 were prescribed evogliptin at a dose of 5 mg, 139 were prescribed sitagliptin at a dose of 100 mg. After 24 weeks The absolute change in HbA1c levels compared to baseline data was analyzed. In the evogliptin group, HbA1c levels decreased by 0.58±0.70% (p<0.001), and in the sitagliptin group - by 0.61±0.66% (p<0.001). The difference in effectiveness between groups is 0.03% (95% confidence interval - 0.14, 0.19%). The data obtained showed that evogliptin was no less effective than sitagliptin. The frequency of adverse events was also comparable between groups (p>0.05). Severe hypoglycemia was not recorded in any of the groups. Over the entire observation period, the development of mild hypoglycemia was even more common in the sitagliptin group than in the evogliptin group, amounting to 5.2% and 0.7%, respectively (p = 0.365) (Fig. 4).
A retrospective study of the database of the clinical trial EVOCOMBI allowed us to identify a number of possible predictors of the effectiveness of evogliptin: a high HOMA-B index (p = 0.0042), a low metabolic index - the ratio of concentrations of triglycerides and high-density lipoproteins (p = 0.0057), low normal concentration of phosphorus in blood plasma (p = 0.014). At the same time, the metabolic index showed itself to be a predictor specific to the Russian population [36].
In the multicenter, randomized, double-blind, active-controlled EVERGREEN trial, the glucose-lowering effect (including glycemic variability) and tolerability of evogliptin were comparable to the efficacy and tolerability of linagliptin [37]. The study involved 207 patients. After 12 weeks treatment, the mean change in HbA1c levels in the evogliptin group and in the linagliptin group was -0.85% and -0.75%, respectively. The change in mean amplitude of glycemic fluctuations was -24.6 mg/dL in the evogliptin group and -16.7 mg/dL in the linagliptin group. These values were significantly lower than the baseline values in both groups, however, they did not differ significantly from each other. In the evogliptin group, HbA1c levels decreased by -0.94% at week 24, reaching values of <7.0% in 80.2% of patients. The incidence and types of adverse events were comparable between the two groups at 24 weeks. Evogliptin therapy improved glycemic variability without causing serious side effects in patients with type 2 diabetes.
The data obtained demonstrate that today it is possible to use a drug from the DPP-4 i group, the effectiveness and safety of which is not inferior to those of other representatives of the group, and the affordability is comparable to the availability of PSM.