Biosulin® R
Target blood glucose concentrations, the insulin preparations to be used, and the insulin dosing regimen (dose and timing of administration) must be determined and adjusted individually to suit the patient's diet, physical activity level, and lifestyle.
The drug Biosulin® R is intended for intravenous, intramuscular and subcutaneous administration.
The dose and route of administration of the drug is determined by the doctor individually in each case based on the concentration of glucose in the blood.
On average, the daily dose of the drug ranges from 0.5 to 1 IU/kg body weight (depending on the individual characteristics of the patient and blood glucose concentration).
The drug is administered 30 minutes before a meal or light snack containing carbohydrates. The temperature of the administered insulin should be at room temperature.
When monotherapy with the drug, the frequency of administration is 3 times a day (if necessary, up to 5-6 times a day). With a daily dose exceeding 0.6 IU/kg, it must be administered in the form of 2 or more injections into different areas of the body.
The doctor should give the necessary instructions on how often to determine the concentration of glucose in the blood, and also give appropriate recommendations in case of any changes in diet or insulin regimen.
In the treatment of severe hyperglycemia, or ketoacidosis in particular, insulin administration is part of a comprehensive treatment regimen that includes measures to protect patients from possible serious complications due to a rapid decrease in blood glucose concentrations. This treatment regimen requires careful monitoring in the intensive care unit (determining metabolic status, acid-base balance and electrolyte balance, monitoring vital signs of the body).
Switching from another type of insulin to Biosulin® R
When switching patients from one type of insulin to another, adjustments to the insulin dosage regimen may be necessary: for example, when switching from animal-derived insulin to human insulin, or when switching from one human insulin preparation to another, or when switching from a treatment regimen with soluble human insulin to a regimen , which includes longer-acting insulin.
After switching from animal-derived insulin to human insulin, a reduction in the insulin dose may be necessary, especially in patients who were previously managed on sufficiently low blood glucose concentrations; in patients prone to developing hypoglycemia; in patients who previously required high doses of insulin due to the presence of antibodies to insulin.
The need for dose adjustment (reduction) may arise immediately after switching to a new type of insulin or develop gradually over several weeks.
When switching from one type of insulin to another and then in the subsequent first weeks, careful monitoring of blood glucose concentrations is recommended. In patients who required high doses of insulin due to the presence of antibodies, it is recommended to switch to another type of insulin under medical supervision in a hospital.
Additional insulin dose changes
Improved metabolic control may lead to increased insulin sensitivity, which may reduce the body's need for insulin.
Dose adjustments may also be necessary if: changes in the patient's body weight, changes in lifestyle (including diet, level of physical activity, etc.) or other circumstances that may increase susceptibility to hypo- or hyperglycemia (see section "Special Instructions" ").
Dosage regimen in certain groups of patients
Elderly patients
In elderly people, the need for insulin may be reduced (see sections “With caution”, “Special instructions”). It is recommended that initiation of treatment, increasing doses and titration of maintenance doses in elderly patients with diabetes mellitus should be carried out with caution to avoid hypoglycemic reactions.
Patients with liver or kidney failure
In patients with liver or kidney failure, the need for insulin may be reduced.
The drug Biosulin® R is usually injected subcutaneously into the anterior abdominal wall. Injections can also be made into the thigh, buttock or shoulder in the projection of the deltoid muscle. It is necessary to change injection sites within the anatomical region to prevent the development of lipodystrophies.
When injecting insulin subcutaneously, care must be taken not to enter a blood vessel when injecting. After the injection, do not massage the injection site. The drug can be administered intramuscularly and intravenously only under the supervision of a physician.
Patients should be trained in the correct use of the insulin delivery device.
Preparing for the introduction
For the drug Biosulin® R in cartridges
Cartridges can only be used if their contents are a clear, colorless liquid without visible particles. Do not use the drug if a precipitate appears in the solution.
The design of the cartridges does not allow mixing their contents with other insulins directly in the cartridge itself. Cartridges are not intended to be refilled.
When using reusable pen cartridges, follow the manufacturer's instructions regarding inserting the cartridge into the pen and attaching the needle. The drug should be administered in accordance with the instructions of the syringe pen manufacturer.
After insertion, the needle should be unscrewed using the outer needle cap and disposed of immediately and safely. Removing the needle immediately after injection ensures sterility and prevents leakage, air entrapment, and possible needle clogging. Then you should put the cap on the pen. Needles should not be reused.
For the drug Biosulin® R in the syringe pen BiomatikPen®2
When using prefilled disposable multi-dose pens for multiple injections, remove the pen from the refrigerator and allow the medication to reach room temperature before first use. You must follow the exact instructions for using the pen supplied with the drug. The drug Biosulin® R in a disposable multi-dose syringe pen cannot be used if it is no longer transparent and colorless, or if it has been frozen.
The drug Biosulin® R in a disposable multi-dose syringe pen and needles are intended for individual use only. The pen cartridge must not be refilled. Needles should not be reused. To protect from light, the syringe pen should be covered with a cap. Do not store a used syringe pen in the refrigerator.
Biosulin® R is a short-acting insulin preparation and is usually used in combination with intermediate-acting insulin (Biosulin® N).
INSTRUCTIONS FOR DRUG INJECTION TECHNIQUE
Biosulin® R injection solution 100 IU/ml
USING A SYRINGE PEN BiomaticPen®2
(single use for multiple injections)
Appearance and parts of the BiomaticPen®2 syringe pen - see fig. “Appearance and parts of the BiomaticPen®2 syringe pen”
Ensuring asepsis during injection
Before giving the injection, you must wash your hands. It is very important that your hands and all equipment needed for injection are clean. Select the injection site. Wipe the skin at the injection site with an alcohol wipe only after the insulin dose has been set in the syringe pen. Before injection, allow the alcohol to dry at the injection site.
Attaching a needle to a syringe pen
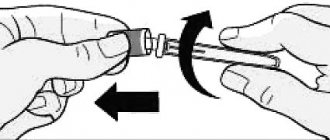
1. Before use, remove the protective cap from the syringe pen. Disinfect the rubber membrane of the cartridge using an alcohol wipe or medical swab soaked in alcohol to prevent the entry of microorganisms (see Figure 1).
2. Remove the protective film from the outer protective cap of the needle (see Fig. 2).
Attention! For each injection, use a new, sterile pen needle (with the protective film intact)!
Read the instructions for using the pen needle!
3. Carefully and tightly screw the needle onto the threads of the cartridge holder tip until it stops (see Fig. 3).
4. Remove the outer needle protective cap and save it for removal and disposal of the used needle (see Figure 4).
5. Remove and discard the inner needle protection cap (see Figure 5).
Attention! The needle of the syringe pen is sterile! Don't touch her!
Use a new needle for each injection to prevent infection.
To avoid accidental needle sticks, never put the inner cap back on the needle.
Preparing a syringe pen for injection
6. Turn the dose setting ring clockwise to set the test dose to 2 units (see Fig. 6).
7. Install the BiomaticPen®2 syringe pen with the working end up and gently tap on the cartridge holder so that all the air contained in the cartridge rises to the top (see Fig. 7).
8. While holding the pen with the needle up, press the start button all the way. The dosage indicator will return to zero (“0” position) (see Fig. 8).
9. A few drops of the drug should appear at the end of the needle. If this does not happen, the operation (steps 6-8) should be repeated. If no drops appear, use a new needle (the needle may become clogged) (see Fig. 9).
Attention! To ensure that the dose is complete, you should always check that a drop of liquid comes out of the needle before each dose. Setting the required dose of the drug
10. Make sure the dosage indicator is in the “0” position. Set the number of units required to inject the drug by turning the dose setting ring clockwise (see the table for converting the BiomaticPen®2 syringe pen dosage indicator readings into the drug dose). The dose can be adjusted by rotating the dose setting ring in any direction until the correct dose is set opposite the dosage indicator (see Fig. 10).
Attention! When rotating the dose setting ring, be careful not to accidentally press the start button to avoid releasing the dose of the drug.
Safety limiter
The dose of the drug, which is installed on the BiomaticPen®2 syringe pen, can be limited by the amount remaining in the cartridge. If the amount of drug remaining in the cartridge is not sufficient for the required dose, the dose setting ring will not turn further in the clockwise direction. Throw away the pen or administer the remaining dose units and use a new pen to complete the required dose.
Carrying out the injection
11. The injection of the drug should be carried out in accordance with the recommendations of the attending physician.
To administer the set dose of the drug, press the start button all the way and hold it down throughout the entire administration process until the value “0” appears opposite the dosage indicator. The value “0” in the dosage indicator window means that you have administered your dose completely (see Fig. 11).
Note: You can interrupt the injection of the drug by releasing the trigger button.
The amount of drug that was not administered will be displayed in the dosage indicator window and can be additionally entered by pressing the start button again.
12. After injection, the needle should remain under the skin for at least 6 seconds. You should keep the button pressed until the needle is completely removed from under the skin, thus ensuring the correct dose is administered and limiting the possibility of blood or lymph getting into the needle or into the insulin cartridge (see Fig. 12).
Attention!
Failure to follow these steps may result in the wrong dose being administered. If insulin continues to leak from the needle after an injection, hold the needle in the skin longer for subsequent injections.
13. After removing the needle from under the skin, carefully place the outer protective cap on the needle of the syringe pen (see Fig. 13).
Disposal of a needle and storage of a syringe pen
14. Disconnect the needle by turning it counterclockwise and dispose of it properly (see Figure 14).
Attention!
Strictly follow safety precautions to avoid accidental needlestick injury and possible transmission of infectious diseases.
15. Close the syringe pen with a protective cap after each use to prevent direct sunlight and dust from entering the cartridge.
Additional Information
Sound and tactile signals
During operation, the BiomaticPen®2 syringe pen produces the following sound and tactile signals:
— setting the required dose
When rotating the dose setting ring, a certain physical resistance is felt and clicks are heard as each dose unit is dialed.
- injection
The process of administering a drug from a BiomaticPen®2 syringe pen is accompanied by
is given by a sound signal (ratchet), which stops when the drug is completely introduced (up to the “O” value in the dosage indicator window).
Rules for storage, use and disposal
The syringe pen is intended for individual use and cannot be used by several people.
Handle the BiomaticPen®2 syringe pen carefully.
Do not allow dust or moisture to get into the BiomatikPen82 syringe pen.
After each use, close the syringe pen with the cap. Always keep the BiomaticPen®2 syringe pen in its individual packaging without a needle.
Store the BiomaticPen®2 syringe pen following the instructions for storing the medicinal product.
You can clean the BiomaticPen®2 syringe pen with a damp cloth. Do not use alcohol, solvents or other cleaning agents.
Never immerse the BiomaticPen®2 syringe pen in water, as this may damage it.
Warnings
Use the BiomaticPen®2 syringe pen only with pen-compatible needles recommended by your doctor.
Biosulin® R should be used only as part of therapy prescribed by your doctor and in the dosage prescribed for you. Any changes should be made under the supervision of a physician.
If you have questions regarding needle length, consult your doctor or healthcare team.
Do not expose the BiomaticPen®2 syringe pen to extreme temperatures, do not leave it in direct sunlight or in the cold (for example, in the freezer).
Keep the BiomaticPen®2 pen and pen needles out of the reach of children and others who are not familiar with proper handling techniques. In cases of unintentional administration of the drug or injury from a needle stick, you should immediately seek medical help!
Pen needles should only be used by one person to prevent the transmission of infectious diseases.
Use a new pen needle for each injection to ensure sterility. Remove the pen needle after injecting to prevent drug leakage, air ingress, and possible clogging of the pen needle.
Dispose of used pen needles together with their protective cap, following the manufacturer's instructions, so that they do not cause harm to others.
Never use the BiomaticPen®2 syringe pen if you have any doubts about its correct operation.
Disposal rules
The BiomaticPen®2 syringe pen does not contain components that are hazardous to the environment and can be disposed of with normal household waste.
The used BiomaticPen®2 syringe pen should be disposed of only with the needle disconnected.
Table of conversion of the dosage indicator readings of the BiomaticPen®2 syringe pen into the dose of the drug Biosulin® R, injection solution 100 IU/ml:
| Dosage indicator indications | Dose of Biosulin® R, ME |
| 01 | 01 |
| 02 | 02 |
| 03 | 03 |
| 04 | 04 |
| 05 | 05 |
| 06 | 06 |
| 07 | 07 |
| 08 | 08 |
| 09 | 09 |
| 10 | 10 |
| 11 | 11 |
| 12 | 12 |
| 13 | 13 |
| 14 | 14 |
| 15 | 15 |
| 16 | 16 |
| 17 | 17 |
| 18 | 18 |
| 19 | 19 |
| 20 | 20 |
| 21 | 21 |
| 22 | 22 |
| 23 | 23 |
| 24 | 24 |
| 25 | 25 |
| 26 | 26 |
| 27 | 27 |
| 28 | 28 |
| 29 | 29 |
| 30 | 30 |
| 31 | 31 |
| 32 | 32 |
| 33 | 33 |
| 34 | 34 |
| 35 | 35 |
| 36 | 36 |
| 37 | 37 |
| 38 | 38 |
| 39 | 39 |
| 40 | 40 |
| 41 | 41 |
| 42 | 42 |
| 43 | 43 |
| 44 | 44 |
| 45 | 45 |
| 46 | 46 |
| 47 | 47 |
| 48 | 48 |
| 49 | 49 |
| 50 | 50 |
| 51 | 51 |
| 52 | 52 |
| 53 | 53 |
| 54 | 54 |
| 55 | 55 |
| 56 | 56 |
| 57 | 57 |
| 58 | 58 |
| 59 | 59 |
| 60 | 60 |
Instructions to be given to the patient
Injection technique when using insulin in vials
If the patient is using only one type of insulin
1. Disinfect the rubber membrane of the bottle.
2. Fill the syringe with air in a volume corresponding to the required dose of insulin. Inject air into the insulin vial.
3. Turn the bottle with the syringe upside down and draw the required dose of insulin into the syringe. Remove the needle from the vial and remove air from the syringe. Check that your insulin dose is correct.
4. Inject immediately.
If a patient needs to mix two types of insulin
1. Disinfect the rubber membranes of the bottles.
2. Immediately before drawing, roll the vial of long-acting insulin (“cloudy”) between your palms until the insulin becomes uniformly white and cloudy.
3. Fill the syringe with air in a volume corresponding to the dose of “cloudy” insulin.
Inject air into the vial of cloudy insulin and remove the needle from the vial.
4. Fill the syringe with air in a volume corresponding to the dose of short-acting (“transparent”) insulin. Inject air into the bottle with “transparent” insulin.
Turn the bottle with the syringe upside down and draw the required dose of “transparent” insulin. Remove the needle and remove air from the syringe. Check the correct dose.
5. Insert the needle into the vial with “cloudy” insulin, not
Biosulin R, 5 pcs., 3 ml, 100 IU/ml, solution for injection
S/c, i/m, i/v,
30 minutes before a meal or light snack containing carbohydrates.
The dose of the drug is determined by the doctor individually, in each specific case, based on the level of glucose in the blood.
On average, the daily dose is from 0.5 to 1 IU/kg body weight (depending on the individual characteristics of the patient and the level of glucose in the blood).
The temperature of the administered insulin should be at room temperature.
For monotherapy, the frequency of administration is 3 times a day (if necessary, 5-6 times a day). With a daily dose exceeding 0.6 IU/kg, it must be administered in the form of 2 or more injections into different areas of the body.
Biosulin® R is usually administered subcutaneously into the anterior abdominal wall. Injections can also be given in the thigh, buttock or deltoid muscle area. It is necessary to change injection sites within the anatomical region to prevent the development of lipodystrophy.
Biosulin® R can be administered intramuscularly and intravenously only under the supervision of a physician.
Biosulin® R is a short-acting insulin and is usually used in combination with intermediate-acting insulin (Biosulin® N).
Injection technique when using insulin in vials
If the patient is using only one type of insulin
1. The rubber membrane on the bottle should be disinfected.
2. Draw air into the syringe in an amount corresponding to the required dose of insulin. Inject air into the insulin vial.
3. Turn the bottle with the syringe upside down and draw the required dose of insulin into the syringe. Remove the needle from the vial and remove air from the syringe. Check that the insulin dose is set correctly.
4. Inject immediately.
If a patient needs to mix two types of insulin
1. The rubber membranes on the bottles should be disinfected.
2. Immediately before drawing, roll the vial of long-acting insulin (“cloudy”) between your palms until the insulin becomes uniformly white and cloudy.
3. Draw air into the syringe in an amount corresponding to the dose of “cloudy” insulin. Inject air into the bottle with “cloudy” insulin and remove the needle from the bottle (“cloudy” insulin should not be drawn at this stage yet).
4. Draw air into the syringe in an amount corresponding to the dose of short-acting (“transparent”) insulin. Inject air into the vial with “transparent” insulin. Turn the bottle with the syringe upside down and draw the required dose of “transparent” insulin. Remove the needle and remove air from the syringe. Check that the dose taken is correct.
5. Insert the needle into the bottle with “cloudy” insulin, turn the bottle with the syringe upside down and draw the required dose of insulin. Remove air from the syringe and check the correct dose. Immediately inject the collected insulin mixture.
6. Insulins should always be taken in the same sequence described above.
Injection technique when using insulin cartridges
The cartridge with Biosulin® R is intended for use only with the Biosulin Pen syringe pen. The patient should be warned about the need to carefully follow the instructions in the instructions for using the syringe pen for administering insulin.
Before use, make sure that the cartridge with Biosulin® R is not damaged (for example, cracks). Do not use the cartridge if there is any visible damage. After the cartridge is inserted into the pen, a colored stripe should be visible through the window of the cartridge holder.
After injection, the needle should remain under the skin for at least 6 seconds. You should keep the button pressed until the needle is completely removed from under the skin, i.e. ensures the correct dose is administered and limits the possibility of blood or lymph getting into the needle or insulin cartridge.
The cartridge with Biosulin® R is intended for individual use only and cannot be refilled.
Injection procedure
1. Use two fingers to gather a fold of skin, then insert a needle into the base of the fold at an angle of about 45° and inject insulin under the skin.
2. After injection, the needle must remain under the skin for at least 6 seconds to ensure that the insulin is completely injected.
3. If after removing the needle there is blood at the injection site, you must press the injection site with your finger.
4. Injection sites should be changed.