Pharmacological properties of the drug Clexane
Low molecular weight heparin (average molecular weight about 4500 Da), in which the antithrombotic and anticoagulant activities of standard heparin are separated. Unlike standard unfractionated heparin, it is characterized by high anti-Xa activity (100 IU/ml) and weak anti-IIa or antithrombin activity (28 IU/ml). When used in recommended doses, enoxaparin sodium does not increase bleeding time. In prophylactic doses, enoxaparin sodium does not lead to significant changes in activated partial thromboplastin time (aPTT), and also does not affect platelet aggregation and fibrinogen binding to platelets. The pharmacokinetic parameters of the drug are assessed by changes in anti-Xa and anti-IIa activity in blood plasma over time in the recommended dose ranges. When administered subcutaneously, Clexane is quickly and almost completely absorbed. Absorption is directly proportional to the administered dose and is linear. The bioavailability of enoxaparin sodium when administered subcutaneously approaches 100%. Maximum anti-Xa activity in blood plasma is observed between the 3rd and 5th hour after subcutaneous administration and averages 0.18±0.04 IU/ml after administration of 2000 anti-Xa IU and 0. 43±0.11 IU/ml after administration of 4000 anti-Xa IU, and 1.01±0.14 IU/ml after administration of 10,000 anti-Xa IU. Maximum anti-IIa activity is observed on average 4 hours after subcutaneous administration at a dose of 4000 anti-Xa IU, while at the same time, when administered at a dose of 2000 anti-Xa IU, this activity cannot be determined by the traditional amidolytic method. The volume of distribution of enoxaparin sodium for anti-Xa activity almost corresponds to the volume of circulating blood. Metabolism of enoxaparin sodium occurs in the liver by desulfation and/or depolymerization to form low molecular weight heparin species with significantly lower biological potential. The half-life of anti-Xa activity corresponds to approximately 4 hours with a single dose and 7 hours with repeated administration. Anti-Xa activity is detectable up to approximately 24 hours after subcutaneous administration of 4000 anti-Xa IU enoxaparin sodium. Renal clearance of active metabolites is 10%, total renal excretion is 40% of the drug dose. Elimination of enoxaparin is longer in the elderly (half-life is 6–7 hours). In patients with renal failure (creatinine clearance ≤30 ml/min), AUC increases significantly (by 65%) with repeated administration of 4000 anti-Xa IU once daily. Pharmacokinetic parameters in patients with renal failure on hemodialysis do not change.
Composition, manufacturer and release form of Clexane
The main active ingredient in Klesan is enoxaparin. This is a low molecular weight heparin, the relative molecular weight of which is 4500 daltons. The drug is obtained by alkaline hydrolysis of heparin benzyl ether from the mucous membranes of the pig intestine.
As a preventive measure, the use of Clexane practically does not change the speed and quality of blood clotting. When the dose is increased to a therapeutic dose, the APTT increases on average by 2 times.
The drug is produced in French. Clexane is an injection solution. It is colorless, transparent, a slight yellowish tint is allowed.
The substance is packaged in sealed sterile disposable syringes. The dosage may vary depending on the number of active units included:
- 10,000 anti-Ha IU;
- 8000 anti-Ha ME;
- 6000 anti-Ha IU;
- 2000 anti-Ha ME.
According to the concentration level, the syringe contains the following volume:
- 1 ml;
- 0.8 ml;
- 0.6 ml;
- 0.2 ml.
The package contains from one to five blisters, each of which contains two syringes.
Indications for use of the drug Clexane
Prevention of venous thrombosis and embolism during orthopedic or general surgical operations, as well as in therapeutic patients on bed rest due to acute illnesses (heart failure of functional class III–IV according to the NYHA classification, respiratory failure, severe acute infectious process, rheumatic diseases) ; prevention of thrombus formation in the extracorporeal circuit during hemodialysis; treatment of deep vein thrombosis, including those accompanied by pulmonary embolism; treatment of unstable angina and acute myocardial infarction without a pathological Q (in combination with acetylsalicylic acid).
Interaction
Clexane® should not be mixed with other drugs.
When used simultaneously with drugs that affect hemostasis (systemic salicylates, acetylsalicylic acid, NSAIDs (including ketorolac), dextran with a molecular weight of 40 kDa, ticlopidine and clopidogrel, systemic corticosteroids, thrombolytics or anticoagulants, other antiplatelet drugs (including glycoprotein IIb antagonists) IIIa), the risk of bleeding increases (see “Special Instructions”).
Use of the drug Clexane
The drug is used only in adults. For prophylactic and therapeutic use, enoxaparin is administered deeply subcutaneously. The drug is administered intravenously to achieve anticoagulation during hemodialysis. Enoxaparin cannot be administered intramuscularly! Clexane is injected into the antero- or posterolateral area of the abdominal wall. The syringe needle is inserted over its entire length in a perpendicular direction to the surface of the skin fold, which is formed using the thumb and index finger and held throughout the entire injection. The patient should be in a supine position. 1 mg of enoxaparin sodium (0.01 ml solution) corresponds to approximately 100 anti-Xa IU activity. For the prevention of venous thrombosis and thromboembolism during operations with a moderate risk of thrombus formation (abdominal surgery) and in patients with a moderate risk of thromboembolism, the drug is recommended to be administered subcutaneously at a dose of 2000 anti-Xa IU once a day. For operations with a high risk of thromboembolism (surgeries on the hip or knee joint and oncological interventions), the drug is administered subcutaneously at a dose of 4000 anti-Xa IU once a day. In general surgical practice, the first dose of the drug is administered 2 hours before surgery. In orthopedic practice, the first dose of the drug is administered 12 hours before surgery. The duration of prophylactic use is on average 7–10 days. In orthopedics, it is used in a dose of 4000 anti-Xa once a day for up to 4 weeks. In immobilized medical patients at high risk of thromboembolism, the recommended dose is 4000 anti-Xa IU once daily for at least 6 days, but not more than 14 days. To prevent thrombus formation in the extracorporeal circuit during hemodialysis, the drug is used at a dose of 100 anti-Xa IU/kg of patient body weight. Enoxaparin is injected into the arterial line of the hemodialysis circuit before the start of the session. As a rule, the indicated dose is sufficient for dialysis for 4 hours; if fibrin rings appear, an additional dose of 50–100 anti-Xa IU/kg may be administered. For patients with a high risk of bleeding, the dose of the drug should be reduced to 50 anti-Xa IU/kg with dual vascular access and to anti-Xa IU/kg with single access. When fibrin rings appear, an additional dose of 50 to 100 anti-Xa IU/kg is administered. In the treatment of deep vein thrombosis, which is accompanied or not accompanied by pulmonary embolism, enoxaparin sodium is administered subcutaneously at a dose of 150 anti-Xa IU/kg once a day or at a dose of 100 anti-Xa IU/kg 2 times a day every 12 h. The duration of treatment should not exceed 10 days. If necessary, oral anticoagulants are simultaneously prescribed. Treatment is continued until the international normalization ratio (INR) reaches 2–3. Q wave in the acute phase, enoxaparin sodium is administered subcutaneously at a dose of 100 anti-Xa IU/kg every 12 hours (in combination with acetylsalicylic acid at a dose of 100–325 mg orally once a day) . The duration of treatment is 2–8 days until the patient’s condition is clinically stabilized. There is no need for dose adjustment in elderly patients with normal renal function. The use of enoxaparin sodium in children is not recommended. In patients with renal failure (creatinine clearance ≤30 ml/min), dose adjustment of the drug is required, since its effect in this category of patients is significantly enhanced. For prophylactic purposes, such patients are prescribed the drug in a dose of 20 mg (2000 anti-Xa IU) once a day, for therapeutic purposes - 1 mg/kg (100 anti-Xa IU/kg) once a day. Administration of the drug to patients with liver failure requires medical supervision.
Clexane®
Subcutaneously, except in special cases (see below subsections “Treatment of ST-segment elevation myocardial infarction, drug or percutaneous coronary intervention” and “Prevention of thrombus formation in the extracorporeal circulation during hemodialysis”).
Prevention of venous thrombosis and embolism during surgical interventions in patients at moderate and high risk
For patients with a moderate risk of developing thrombosis and embolism (for example, abdominal surgery), the recommended dose of Clexane® is 20 mg once daily subcutaneously. The first injection should be given 2 hours before surgery.
Patients with a high risk of developing thrombosis and embolism (for example, during orthopedic operations, surgical operations in oncology, patients with additional risk factors not related to the operation, such as congenital or acquired thrombophilia, malignancy, bed rest for more than three days, obesity, venous history of thrombosis, varicose veins of the lower extremities, pregnancy) the drug is recommended at a dose of 40 mg once a day subcutaneously, with the first dose administered 12 hours before surgery. If earlier preoperative prophylaxis is necessary (for example, in patients at high risk of developing thrombosis and thromboembolism awaiting delayed orthopedic surgery), the last injection should be given 12 hours before surgery and 12 hours after surgery.
The average duration of treatment with Clexane® is 7-10 days. If necessary, therapy can be continued as long as the risk of thrombosis and embolism remains, and until the patient switches to an outpatient regimen.
For major orthopedic operations, it may be advisable to continue treatment after initial therapy by administering Clexane® at a dose of 40 mg once daily for five weeks.
For patients at high risk of venous thromboembolism who have undergone surgery, abdominal and pelvic surgery due to cancer, it may be advisable to increase the duration of administration of Clexane® at a dose of 40 mg once daily for four weeks.
Prevention of venous thrombosis and embolism in patients on bed rest due to acute therapeutic diseases
The recommended dose of Clexane® is 40 mg once a day, subcutaneously, for 6-14 days. Therapy should be continued until the patient is completely outpatient (maximum 14 days).
Treatment of deep vein thrombosis with or without pulmonary embolism
The drug is administered subcutaneously at the rate of 1.5 mg/kg body weight once a day or 1 mg/kg body weight twice a day. The dosage regimen should be selected by the physician based on an assessment of the risk of thromboembolism and the risk of bleeding. In patients without thromboembolic complications and with a low risk of developing venous thromboembolism, the drug is recommended to be administered subcutaneously at a dose of 1.5 mg/kg body weight once a day. In all other patients, including patients with obesity, symptomatic pulmonary embolism, cancer, recurrent venous thromboembolism and proximal thrombosis (iliac vein), the recommended dose is 1 mg/kg twice daily.
The average duration of treatment is 10 days. Therapy with indirect anticoagulants should be started immediately, and treatment with Clexane must be continued until a therapeutic anticoagulant effect is achieved (INR [International Normalized Ratio] values should be 2.0-3.0).
Prevention of thrombus formation in the extracorporeal circulation system during hemodialysis
The recommended dose of Clexane® is on average 1 mg/kg body weight. If there is a high risk of bleeding, the dose should be reduced to 0.5 mg/kg body weight with double vascular access or to 0.75 mg/kg with single vascular access.
During hemodialysis, Clexane® should be injected into the arterial site of the shunt at the beginning of the hemodialysis session. One dose is usually sufficient for a four-hour session, however, if fibrin rings are detected during longer hemodialysis, you can additionally administer the drug at the rate of 0.5-1 mg/kg body weight. There are no data available for patients using enoxaparin sodium for prophylaxis or treatment and during hemodialysis sessions.
Treatment of unstable angina and non-ST segment elevation myocardial infarction
The drug Clexane® is administered at the rate of 1 mg/kg body weight every 12 hours, subcutaneously, while using antiplatelet therapy. The average duration of therapy is at least 2 days and continues until the patient's clinical condition has stabilized. Typically, the administration of the drug lasts from 2 to 8 days. Acetylsalicylic acid is recommended for all patients who do not have contraindications, with an initial dose of 150-300 mg orally, followed by a maintenance dose of 75-325 mg once daily.
Treatment of acute ST-segment elevation myocardial infarction, medical or percutaneous coronary intervention
Treatment begins with a single intravenous bolus of enoxaparin sodium at a dose of 30 mg. Immediately after this, enoxaparin sodium is administered subcutaneously at a dose of 1 mg/kg body weight. Next, the drug is administered subcutaneously at 1 mg/kg body weight every 12 hours (maximum 100 mg of enoxaparin sodium for each of the first two subcutaneous injections, then 1 mg/kg body weight for the remaining subcutaneous doses, that is, for body weight more than 100 kg, a single dose cannot exceed 100 mg). As soon as possible after the diagnosis of acute ST-segment elevation myocardial infarction, patients should be prescribed concomitant acetylsalicylic acid and, unless contraindicated, acetylsalicylic acid (in doses of 75-325 mg) should be continued daily for at least 30 days.
The recommended duration of treatment with Clexane® is 8 days or until the patient is discharged from the hospital (if the period of hospitalization is less than 8 days).
When combined with thrombolytics (fibrin-specific and fibrin-nonspecific), enoxaparin sodium should be administered between 15 minutes before the start of thrombolytic therapy and up to 30 minutes after it.
In patients aged 75 years and older, the initial intravenous bolus is not used. The drug is administered subcutaneously at a dose of 0.75 mg/kg every 12 hours (maximum 75 mg of enoxaparin sodium for each of the first two subcutaneous injections, then 0.75 mg/kg body weight for the remaining subcutaneous doses, that is, for body weight more than 100 kg, single dose cannot exceed 75 mg).
In patients undergoing percutaneous coronary intervention, if the last subcutaneous injection of enoxaparin sodium was given less than 8 hours before inflation of the balloon catheter inserted into the site of narrowing of the coronary artery, additional administration of enoxaparin sodium is not required. If the last subcutaneous injection of enoxaparin sodium was administered more than 8 hours before inflation of the balloon catheter, an additional intravenous bolus of enoxaparin sodium should be administered at a dose of 0.3 mg/kg.
Features of drug administration
The pre-filled disposable syringe is ready for use.
The drug cannot be administered intramuscularly!
Subcutaneous administration
It is advisable to carry out injections with the patient lying down. When using pre-filled 20 mg and 40 mg syringes, do not remove air bubbles from the syringe before injection to avoid loss of the drug. Injections should be performed alternately into the left or right anterolateral or posterolateral surface of the abdomen.
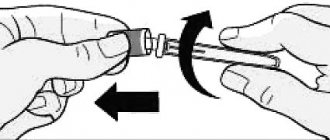
The needle must be inserted to its full length, vertically (not from the side), into a skin fold, collected and held until the injection is completed between the thumb and forefinger. The skin fold is released only after the injection is completed.
Do not massage the injection site after administering the drug.
Intravenous bolus
An intravenous bolus of enoxaparin sodium should be administered through a venous catheter. Enoxaparin sodium should not be mixed or administered with other drugs. In order to avoid the presence of traces of other drugs in the infusion system and their interaction with enoxaparin sodium, the venous catheter should be flushed with a sufficient amount of 0.9% sodium chloride solution or 5% dextrose solution before and after intravenous bolus administration of enoxaparin sodium. Enoxaparin sodium can be safely administered with 0.9% sodium chloride solution and 5% dextrose solution.
To administer a 30 mg bolus of enoxaparin sodium in the treatment of acute ST-segment elevation myocardial infarction, excess drug is removed from 60 mg, 80 mg and 100 mg glass syringes so that only 30 mg (0.3 ml) remains. A dose of 30 mg can be administered directly intravenously.
For intravenous bolus administration of enoxaparin sodium through a venous catheter, pre-filled syringes for subcutaneous administration of the drug 60 mg, 80 mg and 100 mg can be used. It is recommended to use 60 mg syringes as this reduces the amount of drug removed from the syringe. 20 mg syringes are not used because they do not contain enough drug to administer a 30 mg bolus of enoxaparin sodium. 40 mg syringes are not used because they do not have graduations and therefore it is impossible to accurately measure the amount of 30 mg.
To improve the accuracy of additional intravenous bolus administration of small volumes into the venous catheter during percutaneous coronary interventions, it is recommended to dilute the drug to a concentration of 3 mg/ml. It is recommended to dilute the solution immediately before administration.
To obtain a 3 mg/mL enoxaparin sodium solution using a 60 mg prefilled syringe, it is recommended to use a 50 mL infusion solution container (i.e., 0.9% sodium chloride solution or 5% dextrose solution). 30 ml of solution is removed and removed from the container with the infusion solution using a regular syringe. Enoxaparin sodium (contents of the syringe for subcutaneous administration is 60 mg) is injected into the remaining 20 ml of infusion solution in the container. The contents of the container with a diluted solution of enoxaparin sodium are carefully mixed. For administration, the required volume of a diluted solution of enoxaparin sodium is extracted using a syringe, which is calculated using the formula:
Volume of diluted solution = Patient’s body weight (kg) x 0.1
or using the table below.
Volumes to be administered intravenously after dilution to a concentration of 3 mg/ml
| Patient's body weight, kg | Required dose (0.3 mg/kg), mg | The volume of solution required for administration, diluted to a concentration of 3 mg/ml |
| 45 | 13,5 | 4,5 |
| 50 | 15 | 5 |
| 55 | 16,5 | 5,5 |
| 60 | 18 | 6 |
| 65 | 19,5 | 6,5 |
| 70 | 21 | 7 |
| 75 | 22,5 | 7,5 |
| 80 | 24 | 8 |
| 85 | 25,5 | 8,5 |
| 90 | 27 | 9 |
| 95 | 28,5 | 9,5 |
| 100 | 30 | 10 |
| 105 | 31,5 | 10,5 |
| 110 | 33 | 11 |
| 115 | 34,5 | 11,5 |
| 120 | 36 | 12 |
| 125 | 37,5 | 12,5 |
| 130 | 39 | 13 |
| 135 | 40,5 | 13,5 |
| 140 | 42 | 14 |
| 145 | 43,5 | 14,5 |
| 150 | 45 | 15 |
Switching between enoxaparin sodium and oral anticoagulants
— Switching between enoxaparin sodium and vitamin K antagonists (VKAs)
Monitoring the effect of VKA requires physician supervision and laboratory tests [prothrombin time, reported as INR].
Because the maximum effect of VKAs takes time to develop, therapy with enoxaparin sodium should be continued at a constant dose for as long as necessary to maintain INR values (based on two consecutive determinations) in the desired therapeutic range, depending on the indication.
For patients receiving VKA, discontinuation of VKA and administration of the first dose of enoxaparin sodium should occur after the INR has decreased below the therapeutic range.
— Switching between enoxaparin sodium and direct oral anticoagulants (DOACs)
Discontinuation of enoxaparin sodium and administration of DOACs should be carried out 0-2 hours before the next scheduled administration of enoxaparin sodium in accordance with the instructions for the use of oral anticoagulants.
For patients receiving DOACs, administration of the first dose of enoxaparin sodium and discontinuation of direct oral anticoagulants should be carried out at a time point corresponding to the next planned use of DOACs.
Use during spinal/epidural anesthesia or lumbar puncture
If anticoagulant therapy is used during epidural or spinal anesthesia/analgesia or lumbar puncture, neurological monitoring is necessary due to the risk of developing neuraxial hematomas (see section "Special Instructions").
— Use of enoxaparin sodium in prophylactic doses
Insertion or removal of a catheter should occur at least 12 hours after the last injection of a prophylactic dose of enoxaparin sodium.
When using a continuous technique, an interval of at least 12 hours must be maintained before catheter removal.
In patients with CC ≥15 and <30 ml/min, doubling the time until puncture or catheter insertion/removal should be considered to at least 24 hours. Preoperative administration of enoxaparin sodium 2 hours before surgery at a dosage of 20 mg is incompatible with neuraxial anesthesia .
— Use of enoxaparin sodium in therapeutic doses
Insertion or removal of the catheter should be carried out at least 24 hours after the last injection of a therapeutic dose of enoxaparin sodium (see section "Contraindications").
When using a continuous technique, an interval of at least 24 hours must be maintained before catheter removal.
In patients with CC ≥15 and <30 ml/min, doubling the time until puncture or catheter insertion/removal should be considered to at least 48 hours.
Patients receiving enoxaparin sodium at doses of 0.75 mg/kg or 1 mg/kg body weight twice daily should not receive a second dose of the drug to extend the interval before catheter insertion or replacement. Similarly, the possibility of delaying the next dose of the drug by at least 4 hours should be considered, based on an assessment of the benefit/risk ratio (risk of thrombosis and bleeding during the procedure, taking into account the presence of risk factors in patients). At these time points, anti-Xa activity of the drug is still detectable, and time delays do not guarantee that the development of a neuraxial hematoma can be avoided.
Dosage regimen
in
special groups of patients
Children under 18 years of age
The safety and effectiveness of enoxaparin sodium in children have not been established.
Elderly patients (over 75 years old)
With the exception of the treatment of myocardial infarction with ST segment elevation, for all other indications, dose reduction of enoxaparin sodium in elderly patients in the absence of impaired renal function is not required.
Patients with impaired renal function
— Severe renal impairment (creatinine clearance ≥15 and <30 ml/min)
The use of enoxaparin sodium is not recommended for patients with end-stage chronic kidney disease (creatinine clearance <15 ml/min) due to the lack of data, except in cases of prevention of thrombus formation in the extracorporeal circulation system during hemodialysis.
In patients with severe renal impairment (creatinine clearance ≥15 and <30 ml/min), the dose of enoxaparin sodium is reduced in accordance with the tables below, since these patients experience an increase in systemic exposure (duration of action) of the drug.
When using the drug in therapeutic doses, the following dosage adjustment is recommended:
| Usual dosage regimen | Dosage regimen for severe renal failure |
| 1 mg/kg body weight subcutaneously twice daily | 1 mg/kg body weight subcutaneously once daily |
| 1.5 mg/kg body weight subcutaneously once daily | 1 mg/kg body weight subcutaneously once daily |
| Treatment of acute ST-segment elevation myocardial infarction in patients under 75 years of age | |
| A single intravenous bolus of 30 mg plus 1 mg/kg body weight subcutaneously; followed by subcutaneous administration at a dose of 1 mg/kg body weight twice daily (maximum 100 mg for each of the first two subcutaneous injections) | A single intravenous bolus of 30 mg plus 1 mg/kg body weight subcutaneously; followed by subcutaneous administration at a dose of 1 mg/kg body weight once daily (maximum 100 mg for the first subcutaneous injection only) |
| Treatment of acute ST-segment elevation myocardial infarction in patients aged 75 years and older | |
| 0.75 mg/kg body weight subcutaneously twice daily without an initial intravenous bolus (maximum 75 mg for each of the first two subcutaneous injections) | 1 mg/kg body weight subcutaneously once daily without initial intravenous bolus (maximum 100 mg for first subcutaneous injection only) |
When using the drug for prophylactic purposes, it is recommended to adjust the dosage regimen, presented in the table below.
| Usual dosage regimen | Dosage regimen for severe renal failure |
| 40 mg subcutaneously once daily | 20 mg subcutaneously once daily |
| 20 mg subcutaneously once daily | 20 mg subcutaneously once daily |
The recommended dosage adjustment does not apply to hemodialysis.
— Renal dysfunction of mild (creatinine clearance ≥50 and <80 ml/min) and moderate (creatinine clearance ≥30 and <50 ml/min) severity
No dose adjustment is required, but patients should be under close medical supervision.
Patients with liver dysfunction
Due to the lack of clinical studies, Clexane® should be used with caution in patients with impaired liver function.
Instructions for self-injection of the drug Clexane® (pre-filled syringe with a needle protection system)
1. Wash your hands and the area of skin (injection site) where you will inject the drug with soap and water. Dry them.
2. Take a comfortable sitting or lying position and relax. Make sure that you can clearly see the place where you are going to inject the drug. It is optimal to use a lounge chair, chaise longue or bed covered with pillows for support.
3. Select the injection site on the right or left side of the abdomen. This place should be at least 5 centimeters from the navel towards the sides. Do not self-inject within 5 centimeters of the belly button or around existing scars or bruises. Alternate injection sites on the right and left sides of the abdomen, depending on where you injected the drug the previous time.
4. Wipe the injection site with a swab moistened with alcohol.
5. Carefully remove the cap from the needle of the syringe containing Clexane®. Set the cap aside. The syringe is pre-filled and ready to use. Do not press the plunger to force out air bubbles before inserting the needle into the injection site. This may result in loss of the drug. After removing the cap, do not allow the needle to touch anything. This is necessary to maintain the sterility of the needle.
6. Hold the syringe in the hand with which you are writing, as you would hold a pencil, and with the other hand, gently squeeze the alcohol-wiped injection site between the thumb and forefinger so as to form a fold of skin. Hold the skin fold the entire time you inject the drug.
7. Hold the syringe with the needle pointing down (vertical at a 90° angle). Insert the entire length of the needle into the skin fold.
8. Press the plunger with your finger. This will ensure that the drug is injected into the subcutaneous fatty tissue of the abdomen. Hold the skin fold the entire time you inject the drug.
9. Remove the needle by pulling it back without deviating from its axis. The safety mechanism will automatically close the needle. You can now stop holding the skin fold. System b
Side effects of the drug Clexane
Hemorrhagic complications are possible (including isolated cases of massive bleeding, in particular retroperitoneal and intracranial; some of these cases were fatal); local or generalized allergic reactions; thrombocytopenia (mild, transient, asymptomatic thrombocytopenia in the first days of therapy; immunoallergic thrombocytopenia with thrombosis, which in some cases was complicated by organ infarction or limb ischemia); with prolonged treatment (more than 5 weeks) - early development of osteoporosis; increased activity of transaminases in blood serum; the development of neuraxial hematomas when using enoxaparin during epidural or spinal anesthesia in some cases can lead to neurological disorders of varying severity, including the formation of prolonged or permanent paralysis; reactions at the injection site (from mild irritation to pain, bruising and hematomas at the injection site, in exceptional cases - skin necrosis); skin bullous rashes or systemic allergic reactions, including anaphylactoid. If such side effects occur, treatment with the drug must be stopped. Isolated cases of hypersensitivity with cutaneous vasculitis have been reported; asymptomatic and reversible increase in platelet count and increase in liver enzyme activity.
Special instructions for the use of the drug Clexane
Low molecular weight heparins are not interchangeable drugs, since they differ in molecular weight, specific activity against factor Xa, and dosage regimen. Clexane, like other anticoagulants, should be used with caution in conditions that are accompanied by an increased risk of bleeding, namely: with hemostasis disorders, a history of peptic ulcers, recent hemorrhagic stroke, uncontrolled severe hypertension (arterial hypertension), diabetic retinopathy, neurosurgical or ophthalmic surgical interventions, simultaneous use of drugs that affect hemostasis. During prophylactic treatment of patients over 65 years of age, no increased bleeding was observed, however, when using the drug in therapeutic doses, there may be a risk of developing hemorrhagic complications. Since there are not enough controlled clinical studies in pregnant women, enoxaparin sodium should be prescribed during pregnancy only if there is a vital indication. It is not recommended to use Clexane for the treatment of pregnant women with prosthetic heart valves. It is recommended to stop breastfeeding during treatment with the drug. Not used in pediatric practice. In patients with low body weight (less than 45 kg in women and 57 kg in men), the risk of developing hemorrhagic complications increases, which requires monitoring the patient. In order to reduce the risk of bleeding after percutaneous coronary angioplasty, the catheter providing vascular access should be removed no earlier than 6–8 hours after subcutaneous administration of enoxaparin. The next dose of enoxaparin can be administered only 6–8 hours after removal of the catheter. When performing spinal or epidural anesthesia with the use of enoxaparin sodium at a dose of 4000 anti-Xa IU/kg once a day, cases of the development of neuraxial hematomas and associated neurological disorders were rarely observed. The risk of developing such complications increases with the use of enoxaparin sodium in high doses, the use of permanent postoperative epidural catheters, or the simultaneous use of drugs that affect hemostasis, in particular NSAIDs, during repeated punctures. When combined with spinal or epidural anesthesia and enoxaparin, it is best to install and remove the catheter before administering enoxaparin. When performing spinal or epidural anesthesia, it is best to insert and remove the catheter when the anticoagulant effect of enoxaparin sodium is low: 10–12 hours after administration of a dose of 4000 anti-Xa IU/kg or less, or 24 hours after administration of the drug in high doses (100 anti-Xa IU/kg 2 times a day or 150 anti-Xa IU/kg once a day). The next administration of enoxaparin sodium should be carried out no earlier than 2 hours after removal of the catheter. Strict medical monitoring of the patient's neurological status is necessary. If signs of a spinal hematoma appear, appropriate treatment should be immediately prescribed (if necessary, spinal cord decompression). Medical supervision is required when prescribing the drug to patients with a history of heparin-induced thrombocytopenia with or without thrombosis. It is recommended to determine the platelet count before and throughout the course of treatment. If the platelet count decreases by 30–50% of the initial value, the drug should be discontinued immediately. Enoxaparin sodium in doses used for the prevention of venous thromboembolism does not significantly affect bleeding time and other parameters of blood coagulation, including platelet aggregation or fibrinogen binding to platelets. When using the drug in higher doses, the aPTT and activated clot formation time may increase. However, the increase in these indicators does not directly depend on the increase in the antithrombotic activity of enoxaparin and does not require constant monitoring.
Clexane injections: what are they prescribed for?
Clexane is administered subcutaneously. This method of administration allows for 100% bioavailability of the active substance. Metabolism of the substance occurs in the liver through depolymerization and desulfation. The resulting metabolites do not have such high activity as the active substance.
The half-life of the drug occurs 4 hours after a single administration. If several doses have been administered subcutaneously, the half-life can be observed no earlier than after 7 hours. Slightly less than half of the drug is excreted by the kidneys. Because of this, older patients may experience a delay in the drug, since kidney function in people over 60 years of age is most often impaired.
Clexane is prescribed in the following cases:
- If necessary, ensure the prevention of the formation of blood clots in large veins after surgical operations.
- For the treatment of thrombosis.
- To ensure high rheological blood parameters in sedentary patients and those who are forced to adhere to strict bed rest.
- On hemodialysis, when it is necessary to ensure homogeneity of blood flow.
- As a complex treatment after strokes.
- During rehabilitation after a heart attack.
Clexane is especially often prescribed to older patients based on the results of an ultrasound examination of the carotid artery after a micro-stroke.
Drug interactions Clexane
Due to the increased risk of bleeding, Clexane should not be used simultaneously with acetylsalicylic acid and other NSAIDs in high doses, ticlopidine, clopidogrel, dextran 40, corticosteroids, thrombolytics, anticoagulants, and other antithrombotic drugs, including glycoprotein IIb/IIIa antagonists. If it is necessary to use such combinations, careful clinical and laboratory monitoring should be carried out, however, today there is experience in the safe combined use of enoxaparin sodium with the above drugs.
Contraindications
hypersensitivity to enoxaparin sodium, heparin or its derivatives, including other low molecular weight heparins;
active major bleeding, as well as conditions and diseases in which there is a high risk of bleeding: threatened abortion, cerebral aneurysm or dissecting aortic aneurysm (except for surgical intervention), hemorrhagic stroke, uncontrolled bleeding, severe enoxaparin- and heparin-induced thrombocytopenia ;
The use of Clexane® in pregnant women with artificial heart valves is not recommended (insufficient clinical experience with use);
age under 18 years (efficacy and safety have not been established).
With caution: hemostasis disorders (including hemophilia, thrombocytopenia, hypocoagulation, von Willebrand disease, etc.), severe vasculitis; peptic ulcer of the stomach or duodenum or other erosive and ulcerative lesions of the gastrointestinal tract; recent ischemic stroke; uncontrolled severe arterial hypertension; diabetic or hemorrhagic retinopathy; severe diabetes mellitus; recent or proposed neurological or ophthalmological surgery; performing spinal or epidural anesthesia (potential danger of developing a hematoma), spinal puncture (recently performed); recent birth; bacterial endocarditis (acute or subacute); pericarditis or pericardial effusion; renal and/or liver failure; intrauterine contraception (IUC); severe trauma (especially the central nervous system), open wounds on large surfaces; simultaneous use of drugs that affect the hemostatic system.
The company does not have data on the clinical use of Clexane ® for the following diseases: active tuberculosis, radiation therapy (recently undergone).
Clexane overdose, symptoms and treatment
As a specific antidote, slow intravenous administration of protamine sulfate (hydrochloride) is indicated at the rate of 1 mg of protamine per 1 mg of Clexane (if enoxaparin sodium was administered over the previous 8 hours). However, even with the introduction of protamine sulfate in a high dose, the effect of enoxaparin sodium is not completely neutralized (maximum - up to 60%). Since neutralization may be temporary (due to the absorption characteristics of low molecular weight heparins), the dose of protamine must be divided into several injections (from 2 to 4) over 24 hours.
Pharmacokinetics
The pharmacokinetics of enoxaparin in the indicated dosage regimens is linear. Variability within and between patient groups is low. After repeated subcutaneous administration of 40 mg of enoxaparin sodium once a day and subcutaneous administration of enoxaparin sodium at a dose of 1.5 mg/kg once a day in healthy volunteers, the equilibrium concentration is achieved by day 2, with an average area under the pharmacokinetic curve 15% higher than after a single injection. After repeated subcutaneous injections of enoxaparin sodium at a daily dose of 1 mg/kg twice a day, the equilibrium concentration is achieved after 3–4 days, and the area under the pharmacokinetic curve is on average 65% higher than after a single dose and the average Cmax values are, respectively, 1.2 and 0.52 IU/ml.
The bioavailability of enoxaparin sodium after subcutaneous administration, assessed on the basis of anti-Xa activity, is close to 100%.
The volume of distribution of the anti-Xa activity of enoxaparin sodium is approximately 5 liters and approaches the volume of blood.
Enoxaparin sodium is a drug with low clearance. After intravenous administration for 6 hours at a dose of 1.5 mg/kg, the average clearance of anti-Xa in plasma is 0.74 l/h.
The elimination of the drug is monophasic with T1/2 of 4 hours (after a single subcutaneous injection) and 7 hours (after repeated administration of the drug).
Enoxaparin sodium is primarily metabolized in the liver by desulfation and/or depolymerization to form low molecular weight substances with very low biological activity. Renal excretion of active fragments of the drug is approximately 10% of the administered dose and the total excretion of active and inactive fragments is approximately 40% of the administered dose.
There may be a delay in the elimination of enoxaparin sodium in elderly patients as a result of decreased renal function with age.
A decrease in the clearance of enoxaparin sodium was noted in patients with reduced renal function. After repeated subcutaneous administration of 40 mg of enoxaparin sodium once a day, there is an increase in anti-Xa activity, represented by the area under the pharmacokinetic curve in patients with minor (Cl creatinine 50–80 ml/min) and moderate (Cl creatinine 30–50 ml/min) min) renal dysfunction. In patients with severe renal impairment (Cl creatinine <30 ml/min), the area under the pharmacokinetic curve at equilibrium is on average 65% higher with repeated subcutaneous administration of 40 mg of the drug once daily.
In people with excess body weight, with subcutaneous administration of the drug, the clearance is slightly less. If you do not adjust the dose taking into account the patient’s body weight, then after a single subcutaneous injection of 40 mg of enoxaparin sodium, anti-Xa activity will be 50% higher in women weighing less than 45 kg and 27% higher in men weighing less than 57 kg compared to patients with normal average body weight.