Indications
Exjiva medicine is used to prevent the deterioration of bone cells. Similar symptoms occur when:
- frequent fractures;
- exposure to radioactive radiation;
- surgical procedures in patients with serious tumor metastases spreading to the bones.
Pharmacology
Retarder of bone epithelium resorption. Immune cells that produce protective proteins. The drug Denosumab is a human component that has the protective functions of polysaccharides and has the ability to bind the activated centers of molecules with a certain set of antibodies. RANK proteins are found in the body in membrane connections and are capable of dissolution. RANKL is an intermediary at the moment of transformation of the sequence of chemical processes that carry out the resorption of bone epithelium in the presence of huge multinucleated cells. The high energy of the latter causes the breakdown of bones in the presence of tumor foci. Denosumab prevents bone degradation, which is caused by malignant ulcers.
How to use Exjiva
Dose. How to take Exjiva is determined by highly qualified and trained medical personnel. The amount of medication taken is one dose of subcutaneous injection of one hundred and twenty milligrams for 4 weeks. The product is injected into the area of the shoulder, thigh or abdomen. During the therapy period, it is advisable to consume calcium at a rate of at least five hundred milligrams and vitamin D-400 ME.
Before use, Exgiva solution must be checked for the presence of foreign bodies, color changes or turbidity. And for painless administration, it is advisable to heat the drug to ambient temperature. The medication should not be shaken. The needle used for injection must be 27 gauge.
Release form, composition and packaging
Solution for subcutaneous administration
transparent, colorless to light yellow, practically free of visible inclusions.
1 bottle contains:
active substance:
denosumab - 120 mg;
Excipients:
sorbitol (E420) 78.2 mg, glacial acetic acid 1.8 mg, sodium hydroxide to pH 5.2, water for up to 1.7 ml.
1.7 ml - Glass bottles with a volume of 3 ml (1) - cardboard packs with first opening control. 1.7 ml - Glass bottles with a volume of 3 ml (4) - cardboard packs with first opening control.
Contraindications
- severe untreated hypocalcemia;
- pregnancy;
- lactation period;
- age under 18 years (not recommended for use in pediatrics, since the effectiveness and safety of this drug have not been studied in this age group. In experimental animal studies, inhibition of RANK/RANKL led to inhibition of bone growth and impaired teething. Thus , the use of denosumab in children with open growth plates can lead to impaired bone growth and open growth plates and impaired tooth eruption);
- hypersensitivity to the components of the drug.
Dosage
Introduction
Injecting the drug requires prior training.
Dose
The recommended dose of the drug is one subcutaneous injection of 120 mg every 4 weeks in the thigh, shoulder or abdominal area.
During the course of treatment, it is recommended to additionally take calcium supplements in a dose of at least 500 mg and vitamin D-400 ME.
Elderly patients
Based on the available data on the effectiveness and safety of the drug in this age group, no adjustment of the drug dosage regimen is required.
Kidney failure
Based on the available data on the effectiveness and safety of the drug in this group of patients, no adjustment of the drug dosage regimen is required.
In patients with severe renal failure
(creatinine clearance <30 ml/min) or on dialysis there is a high risk of developing hypocalcemia. Such patients need to additionally take calcium supplements and vitamin D.
Liver failure
Efficacy and safety have not been studied.
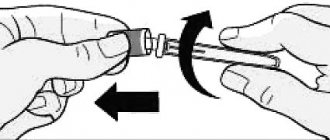
Instructions for use
The solution should be assessed before administration for inclusions or color changes. The solution should not be used if it becomes cloudy or changes color. Do not shake.
It is recommended to administer the drug with a 27-gauge single-use needle.
To avoid discomfort at the injection site, the solution should be warmed to room temperature before injection.
Any unused quantities of the drug or unused materials must be destroyed in accordance with national requirements.
Xgeva
Denosumab is a fully human monoclonal antibody (IgG2) that has high affinity and specificity for receptor activator of nuclear factor kappa B (RANKL) ligand and interferes with the activation of the only RANKL receptor, activator of nuclear factor kB (RANK), located on the surface of osteoclasts and their precursors. The RANK ligand is a protein present in the body in membrane-bound and soluble forms. RANKL is a major mediator of a metabolic pathway required for the formation, function and survival of osteoclasts, the only cell type responsible for bone resorption. Increased osteoclast activity induced by RANKL is a major cause of bone destruction in bone metastases from solid tumors and multiple myeloma. Prevention of RANKL/RANK interaction inhibits osteoclast formation, activation, and survival. As a result, denosumab reduces bone resorption and bone destruction caused by malignant neoplasms.
Pharmacodynamic effects
Denosumab 120 mg SC every 4 weeks resulted in rapid reductions in bone resorption markers (urinary N-telopeptide adjusted for creatinine (uNTX/Cr) and serum 1C-telopeptide) with an average reduction of 82% in uNTX/Cr in a week. Reductions in bone turnover markers remained stable and ranged from 74% to 82% for uNTX/Cr from weeks 2 to 25 of repeated doses of 120 mg every 4 weeks. In 2075 patients with metastatic cancer (breast, prostate, other solid tumors, multiple myeloma) treated with XGIVA, the average reduction in uNTX/Cr was approximately 80% of baseline values over 3 months of treatment. Repeated SC administration of 120 mg every 4 weeks or every 12 weeks resulted in a mean decrease in uNTX/Cr from baseline values after 3-6 months of treatment in patients with metastatic cancer and bone metastases (including patients with multiple myeloma and bone metastases ), previously treated with IV bisphosphonates and an initial uNTX/Cr value of less than 50 nmol/mmol at week 25 of treatment.
Immunogenicity
Clinical studies have not shown the formation of neutralizing antibodies. In less than 1% of patients treated with denosumab, binding but not neutralizing antibodies were detected by sensitive immunoassay. There were no changes in the pharmacokinetic profile, toxic profile or clinical response due to antibody formation.
Clinical effectiveness
Prevention of bone complications (OSCT) in patients with metastatic cancer and bone metastases.
The efficacy and safety of Exgiva in preventing skeletal complications in patients with metastatic cancer and bone metastases was demonstrated in 3 randomized, double-blind, active-controlled studies. Exjiva reduces or prevents the risk of developing OSCT or multiple OSCT (first and subsequent), compared with active control, in patients with malignant tumors that have metastasized to the bone.
Effect on pain
Pain analyzes included changes from baseline in the modified Brief Pain Inventory (BPI-SF), time to worsening pain, moderate to severe pain, improvement, and the proportion of patients meeting these criteria. The mean time to worsening (worsening of pain, greater than 4 scale points or greater than or equal to 2 point increase from baseline) was longer for XGIVA compared to the active control. Time to improvement (i.e., greater than or equal to 2 points of pain improvement from baseline on the BPI-SF) was similar for denosumab and active control across individual studies and integrated analyses.
Overall survival and disease progression
Overall survival remained similar between the denosumab and active control groups for all three studies and in the pooled analysis of study data. In each of the three studies, overall survival was balanced between the denosumab and active control groups in patients with malignancies that had metastasized to bone: breast cancer, prostate cancer, other solid tumors, or multiple myeloma.
Overall survival was higher in the Exgiva group in patients with non-small cell lung cancer and higher in the active control group in patients with multiple myeloma. Overall survival was similar between groups in patients with other solid tumors. In an integrated analysis of data from all three studies, overall survival was similar between the denosumab and active control groups.
Side effects
Infections and infestations:
inflammation of the subcutaneous tissue.
From the immune system:
hypersensitivity reactions.
From the side of metabolism and electrolyte metabolism:
hypocalcemia, hypophosphatemia.
For the skin and subcutaneous tissues:
hyperhidrosis.
From the digestive system:
diarrhea, tooth loss.
From the respiratory system:
dyspnea.
From the musculoskeletal system and connective tissue:
osteonecrosis of the jaw.
Hypocalcemia
In clinical trials in patients with tumors metastasizing to bone, hypocalcemia was more common in the Exjiva group (9.6%) than in the active comparator group (5.0%). Grade 3 decreases in serum calcium concentrations were observed in 2.5% of patients in the Exjiva group and in 1.2% of patients in the active comparator group. A grade 4 decrease in serum calcium concentrations was observed in 0.6% of patients and 0.2% of patients in the active control group.
Osteonecrosis of the jaw
In patients with metastatic cancer, osteonecrosis of the jaw was reported in 1.8% in the denosumab group and 1.3% in the active control group. Clinical symptoms did not differ between subgroups. Among patients with confirmed osteonecrosis of the jaw, the majority (81% in both subgroups) had a history of tooth extraction, poor oral hygiene and/or dental debridement. Most patients received chemotherapy.
Exgiva solution for injection 70 mg/ml syringe 1.7 ml 1 pc
Exgiva 120 mg subcutaneously every 4 weeks resulted in rapid reductions in bone resorption markers (urinary N-telopeptide adjusted for creatinine (uNTX/Cr) and serum 1C-telopeptide) with an average reduction of 82% in uNTX/Cr in within a week. Reductions in bone turnover markers remained stable and ranged from 74% to 82% for uNTX/Cr from weeks 2 to 25 of repeated doses of 120 mg every 4 weeks. In 2075 patients with metastatic cancer (breast, prostate, other solid tumors, multiple myeloma) treated with XGIVA®, the average reduction in uNTX/Cr was approximately 80% of baseline values over 3 months of treatment. Repeated subcutaneous administration of 120 mg every 4 weeks or every 12 weeks resulted in a mean decrease in uNTX/Cr from baseline values after 3 to 6 months of treatment in patients with metastatic cancer and bone metastases (including patients with multiple myeloma and bone metastases), previously receiving intravenous bisphosphonates and an initial uNTX/Cr value of less than 50 nmol/mmol at week 25 of treatment. In a phase 2 study in patients with giant cell tumor of bone who received subcutaneous injections of 120 mg of Exjiva® with loading doses of 120 mg on days 8 and 15 after the starting dose in the first month of treatment and then at a dose of 120 mg once every 4 weeks ( Q4W), starting from the second month of treatment, by week 9 there was an average decrease in uNTx/Cr and sCTx by approximately 80%. Reductions in bone turnover markers were maintained, with mean decreases in uNTx/Cr values from 56% to 77% and sCTx values from 79% to 83% from weeks 5 to 25 of continued 120 mg dosing. Immunogenicity Clinical studies have not demonstrated the formation of neutralizing antibodies. In less than 1% of patients treated with denosumab, binding but not neutralizing antibodies were detected by sensitive immunoassay. There were no changes in the pharmacokinetic profile, toxic profile or clinical response due to antibody formation. Clinical effectiveness Prevention of bone complications (OSCT) in patients with metastatic cancer and bone metastases. The effectiveness and safety of Exjiva® in preventing skeletal complications in patients with metastatic cancer and bone metastases was proven in 3 randomized, double-blind, active-controlled studies. Exjiva® reduces or prevents the risk of developing OSCT or multiple OSCT (first and subsequent), compared with active control, in patients with malignant tumors that have metastasized to the bone. Effect on Pain Pain analyzes included changes from baseline in the modified Brief Pain Inventory (BPI-SF), time to worsening pain, moderate to severe pain, improvement, and the proportion of patients meeting these criteria. The mean time to worsening (increased pain, greater than 4 scale points or greater than or equal to 2 points increase from baseline) was longer for XGIVA® compared to the active control. Time to improvement (i.e., greater than or equal to 2 points of pain improvement from baseline on the BPI-SF) was similar for denosumab and active control across individual studies and integrated analyses. Overall survival and disease progression Overall survival remained similar between the denosumab and active control groups for all three studies and in the pooled analysis of study data. In each of the three studies, overall survival was balanced between the denosumab and active control groups in patients with malignancies that had metastasized to bone: breast cancer, prostate cancer, other solid tumors, or multiple myeloma. Overall survival was higher in the Exjiva group in patients with non-small cell lung cancer and higher in the active control group in patients with multiple myeloma. Overall survival was similar between groups in patients with other solid tumors. In an integrated analysis of data from all three studies, overall survival was similar between the denosumab and active control groups. Treatment of giant cell tumor of bone in adult patients or adolescents with a mature skeleton. The safety and effectiveness of Exjiva® were studied in two open-label, single-arm, phase 2 studies involving 305 patients with unresectable giant cell tumor of bone or in whom surgery was likely to be associated with a severe complication. Patients received 120 mg of Exjiva® subcutaneously every 4 weeks with loading doses of 120 mg on days 8 and 15. Overall, 71.6% of patients responded to Exjiva®. Response rate was defined as at least 90% clearance of giant cells from baseline (or complete clearance of giant cells in cases where giant cells were treated in Treatment of hypercalcemia in malignancy. The safety and efficacy of Exjiva® were studied in an open-label, single-arm, phase 2 study in 33 Adult patients with hypercalcemia from malignant tumors (with or without bone metastases) refractory to treatment with intravenous bisphosphonates.Patients were administered 120 mg of Exjiva® subcutaneously every 4 weeks with additional doses of 120 mg on days 8 and 15 of the first month of treatment. malignancies was defined as an albumin-corrected calcium concentration >12.5 mg/dL (3.1 mmol/L) despite treatment with intravenous bisphosphonates in the last 7 to 30 days.The primary endpoint was the number of patients achieving a response, as defined by as an albumin-corrected serum calcium concentration (CSC) ≤ 11.5 mg/dL (2.9 mmol/L), within 10 days after administration of XGIV®. Exjiva® caused a rapid and sustained decrease in albumin-corrected serum calcium concentrations in most patients, including patients with or without bone metastases.
Drug interactions
No drug interaction studies have been conducted.
In clinical studies, the drug was administered simultaneously with standard antineoplastic therapy, incl. patients previously treated with bisphosphonates.
Concomitant use with chemotherapy or hormone therapy did not change the pharmacokinetics/pharmacodynamics of denosumab, nor did previous IV administration of bisphosphonates.
Pharmaceutical incompatibility
The drug should not be mixed with other drugs.
special instructions
Hypocalcemia
Hypocalcemia must first be corrected by taking calcium and vitamin D supplements in adequate doses before starting denosumab therapy. It is recommended to take calcium and vitamin D supplements during use of the drug, in the absence of hypercalcemia.
Patients with severe renal impairment (creatinine clearance less than 30 ml/min) or on dialysis are at greater risk of developing hypocalcemia. Monitoring calcium concentrations in this subgroup of patients is necessary. If hypocalcemia occurs during treatment, additional short-term calcium supplementation is recommended as needed.
Infections of the skin and appendages
Patients receiving denosumab were more likely to experience infections of the skin and its appendages (mainly inflammation of the subcutaneous tissue), in some cases requiring hospitalization. Such reactions were reported more frequently in the denosumab group (0.9%) than in the comparison group (0.7%). Patients should be instructed to immediately seek medical attention if symptoms and signs of subcutaneous tissue inflammation develop.
Osteonecrosis of the jaw
It is recommended that an examination of the oral cavity and a preventive examination by a dentist before starting therapy in patients at risk of developing osteonecrosis of the jaw. Adequate oral hygiene should be maintained throughout the entire period of treatment with Exgiva. During treatment, invasive dental procedures should be avoided if possible. If such procedures are necessary, the decision should be made together with the attending physician. Patients who develop osteonecrosis of the jaw while using Exjiva should receive adequate dental treatment. An individual assessment of the benefit/risk ratio is required before prescribing Exgiva to patients with irreparable risk factors for the development of osteonecrosis of the jaw and for patients in whom osteonecrosis of the jaw developed while taking the drug.
Impact on the ability to drive vehicles and operate machinery
No studies have been conducted on the ability to drive vehicles and operate machinery.
Exgiva (Xgeva) 120 mg 1.7 ml (70 mg/ml) bottle No. 1 - Instructions
Compound
The main active substance is denosumab.
Other ingredients: glacial acetic acid, sodium hydroxide (to regulate pH), sorbitol (E 420), polysorbate 20, water for injection.
Release form
The medicine is produced in the form of an injection solution. Supplied in packs containing one bottle each.
pharmachologic effect
Bone remodeling is a process in which the body continually removes old bone tissue and replaces it with new bone. It is controlled by different types of cells, primarily osteoblasts (which secrete new bone) and osteoclasts (which break down bone); osteocytes are also present in bone.
Osteoclast precursors, called preosteoclasts, express surface receptors called RANK (receptor activator of nuclear factor kappa B). RANK is a member of the tumor necrosis factor receptor (TNFR) superfamily. RANK is activated by RANKL (RANK ligand), which exists as cell surface molecules on osteoblasts. Activation of RANK by RANKL promotes the maturation of preosteoclasts into osteoclasts. Denosumab inhibits osteoclast maturation by binding to and inhibiting RANKL. This mimics the natural action of osteoprotegerin, an endogenous RANKL inhibitor, which results in decreased concentrations (and possibly decreased avidity) in patients suffering from osteoporosis. This protects the bone from destruction and helps counteract the progression of the disease.
Pharmacokinetics
After subcutaneous administration, bioavailability was 62%.
Denosumab contains only amino acids and carbohydrates as native immunoglobulin and is unlikely to be eliminated by hepatic metabolism. Its metabolism and elimination are expected to follow immunoglobulin elimination pathways, resulting in breakdown into small peptides and individual amino acids.
Indications for use
Indications for use of Exjiva are:
- treatment of osteoporosis in postmenopausal women with an increased risk of fractures (the drug reduces the risk of vertebral fractures, non-vertebral fractures and hip fractures);
- treatment of bone loss through hormonal ablation (i.e., reduction of tumor mass obtained with hormonal drugs) in men with prostate cancer who have an increased risk of fractures (the drug reduces the risk of vertebral fractures);
- prevention of bone complications, such as pathological fractures, the need for bone irradiation, spinal cord compression, the need for bone surgery in adults with metastases of solid tumors to the bone.
Contraindications
The medication is contraindicated in people with hypocalcemia, and sufficient levels of calcium and vitamin D must be achieved before starting therapy with Exjiva.
Adverse reactions
The most common side effects are pain in the joints and muscles of the arms or legs. There is an increased risk of disorders such as cellulite, hypocalcemia (low blood calcium levels), allergic hypersensitivity reactions, osteonecrosis of the jaw, and atypical femur fractures.
Another study showed a significant increase in the incidence of eczema and hospitalizations due to skin infections. It has been suggested that the increase in infections with Exjiva treatment may be related to the role of RANKL in the immune system. RANKL is expressed by T helper cells and is thought to be involved in dendritic cell maturation.
Drug interactions
No interaction studies have been conducted.
Application and dosage
The recommended dose of Xgeva is 120 mg as a single injection under the skin (subcutaneously) once every 4 weeks. The drug is injected into the thigh, abdomen, or shoulder. When treating giant cell tumor of bone, an additional dose is given in the first and second weeks after the first dose.
Overdose
To date, no cases of overdose have been reported.
special instructions
Exjiva works by reducing the hormonal signal that leads to excessive bone loss caused by osteoclasts and is only active in the body for six months. Like bisphosphonates, denosumab appears to increase the risk of osteonecrosis of the jaw (ONJ) after tooth extraction or oral surgery, but unlike a bisphosphonate, the risk drops to zero approximately 6 months after injection. Invasive dental procedures should be avoided during this time.
Use during pregnancy and breastfeeding
No appointment allowed.
Impact on the ability to drive vehicles and operate machinery
No special precautions are required.
Terms of sale
As prescribed by a doctor.
Storage conditions
The medication must be stored in a temperature range from 15 to 25 degrees Celsius, in a place away from children.
Pregnancy and lactation
There are no data on the use of the drug during pregnancy. The drug is not recommended for use in pregnant women.
In toxicological studies in animals, at exposures 9.1 times higher than those recommended for clinical use in humans (120 mg every 4 weeks), denosumab had no effect on fertility or fetal development.
Animal experiments have shown that the absence of RANKL can lead to impaired development of lymph nodes in the fetus, and in the postnatal period can cause impaired teething and bone growth; It is also possible to influence the maturation of the mammary gland, which can lead to weakened lactation. It is unknown whether denosumab is excreted in breast milk. Because denosumab is known to potentially cause adverse reactions in infants, it is necessary to either stop breastfeeding or discontinue the drug.
Exgiva solution for subcutaneous administration 120 mg 70 mg/ml syringe 1.7 ml N1
Action
Exjiva is a bone resorption inhibitor.
Denosumab is a fully human monoclonal antibody (IgG2) that has high affinity and specificity for receptor activator of nuclear factor kappa B (RANKL) ligand and interferes with the activation of the single RANKL receptor, activator of nuclear factor kB (RANK), located on the surface of osteoclasts and their precursors. The RANK ligand is a protein present in the body in membrane-bound and soluble forms. RANKL is a major mediator of a metabolic pathway required for the formation, function and survival of osteoclasts, the only cell type responsible for bone resorption. Increased osteoclast activity induced by RANKL is a major cause of bone destruction in bone metastases from solid tumors and multiple myeloma. Prevention of RANKL/RANK interaction inhibits osteoclast formation, activation, and survival. As a result, denosumab reduces bone resorption and bone destruction caused by malignant neoplasms. Pharmacodynamic Effects Denosumab 120 mg SC every 4 weeks resulted in rapid reductions in bone resorption markers (urinary N-telopeptide corrected for creatinine (uNTX/Cr) and serum 1C-telopeptide) with an average reduction of 82% in uNTX /Cr within a week. Reductions in bone turnover markers remained stable and ranged from 74% to 82% for uNTX/Cr from weeks 2 to 25 of repeated doses of 120 mg every 4 weeks. In 2075 patients with metastatic cancer (breast, prostate, other solid tumors, multiple myeloma) treated with XGIVA, the average reduction in uNTX/Cr was approximately 80% of baseline values over 3 months of treatment. Repeated SC administration of 120 mg every 4 weeks or every 12 weeks resulted in a mean decrease in uNTX/Cr from baseline values after 3-6 months of treatment in patients with metastatic cancer and bone metastases (including patients with multiple myeloma and bone metastases ), previously treated with IV bisphosphonates and an initial uNTX/Cr value of less than 50 nmol/mmol at week 25 of treatment.
Immunogenicity Clinical studies have not demonstrated the formation of neutralizing antibodies. In less than 1% of patients treated with denosumab, binding but not neutralizing antibodies were detected by sensitive immunoassay. There were no changes in the pharmacokinetic profile, toxic profile or clinical response due to antibody formation.
Clinical effectiveness Prevention of bone complications (OSCT) in patients with metastatic cancer and bone metastases. The efficacy and safety of Exgiva in preventing skeletal complications in patients with metastatic cancer and bone metastases was demonstrated in 3 randomized, double-blind, active-controlled studies. Exjiva reduces or prevents the risk of developing OSCT or multiple OSCT (first and subsequent), compared with active control, in patients with malignant tumors that have metastasized to the bone.
Effect on Pain Pain analyzes included changes from baseline in the modified Brief Pain Inventory (BPI-SF), time to worsening pain, moderate to severe pain, improvement, and the proportion of patients meeting these criteria. The mean time to worsening (worsening of pain, greater than 4 scale points or greater than or equal to 2 point increase from baseline) was longer for XGIVA compared to the active control. Time to improvement (i.e., greater than or equal to 2 points of pain improvement from baseline on the BPI-SF) was similar for denosumab and active control across individual studies and integrated analyses.
Overall survival and disease progression Overall survival remained similar between the denosumab and active control groups for all three studies and in the pooled analysis of study data. In each of the three studies, overall survival was balanced between the denosumab and active control groups in patients with malignancies that had metastasized to bone: breast cancer, prostate cancer, other solid tumors, or multiple myeloma. Overall survival was higher in the Exgiva group in patients with non-small cell lung cancer and higher in the active control group in patients with multiple myeloma. Overall survival was similar between groups in patients with other solid tumors. In an integrated analysis of data from all three studies, overall survival was similar between the denosumab and active control groups.
Pharmacokinetics After subcutaneous administration, bioavailability is 62%. When administered subcutaneously, denosumab exhibits nonlinear dose-dependent pharmacokinetics over a wide dose range and a dose-dependent increase in exposure for doses of 60 mg (or 1 mg/kg) and above. Repeated administration of 120 mg every 4 weeks results in a two-fold increase in denosumab serum concentrations, reaching steady state at approximately 6 months of treatment. The mean serum concentration at steady state was 20.6 μg/mL (range, 0.456–56.9 μg/mL). In patients who discontinued treatment, the mean T1/2 was approximately 28 days (range 14-55 days). To assess the influence of demographic factors, pharmacokinetics analysis was performed in populations. The results of the analysis showed that age (18-87 years), race, body weight (36-174 kg), tumor type did not show significant differences in pharmacokinetic parameters. The pharmacokinetics and pharmacodynamics of denosumab were similar in men and women and in patients switched to denosumab from IV bisphosphonate therapy. Denosumab consists of amino acids and carbohydrates, like natural immunoglobulin, and therefore is not metabolized through the hepatic metabolic pathways. Denosumab is metabolized and eliminated presumably by the same mechanisms as immunoglobulins, with degradation to small peptides and amino acids.
Selected patient groups Age does not have a significant effect on the pharmacokinetics of denosumab according to pharmacokinetic analysis in a population of patients from 18 years to 87 years.
Pharmacokinetics in children have not been studied. The pharmacokinetics of denosumab do not depend on race. In a study of 55 patients with varying degrees of renal impairment, including patients on dialysis, the degree of renal impairment had no effect on the pharmacokinetics and pharmacodynamics of denosumab; therefore, no adjustment of the denosumab dosage regimen is required for chronic renal failure. No studies have been conducted to study the effect of liver failure on the pharmacokinetics of denosumab.