Instructions for use of APIDRA®
Apidra® should be administered shortly (0-15 minutes) before or shortly after a meal.
Apidra® should be used in treatment regimens that include intermediate- or long-acting insulin or a basal insulin analogue. The drug can be used in combination with oral hypoglycemic agents.
The dosage regimen for Apidra® is selected individually.
Administration of the drug
The drug Apidra® is administered either by subcutaneous injection or by long-term subcutaneous infusion into the subcutaneous fat using an insulin pump.
Apidra should be injected into the subcutaneous fat of the abdomen, upper arm or thigh, or into the subcutaneous fat of the abdomen as a long-term infusion. The injection and infusion sites should be alternated with each new injection within the recommended areas for subcutaneous administration of the drug. The rate of absorption and therefore the time of onset and duration of action may depend on injection site, physical activity, and other factors. Subcutaneous injection into the abdominal wall provides slightly accelerated absorption compared to other injection sites.
Care should be taken to ensure that the drug does not enter a blood vessel. After the injection, there is no need to massage the injection site. It is necessary to teach the patient the correct injection technique.
Special groups of patients
The pharmacokinetic properties of insulin glulisine are usually preserved in patients with impaired renal function.
. However, insulin requirements may be reduced if renal function is impaired.
Pharmacokinetic properties of insulin glulisine in patients with impaired liver function
have not been studied. In patients with impaired liver function, insulin requirements may be reduced due to a reduced capacity for gluconeogenesis and reduced insulin metabolism.
Pharmacokinetic data in elderly patients
, suffering from diabetes, are insufficient. Impaired renal function may result in decreased insulin requirements.
Clinical information on the use of Apidra in children under 6 years of age
limited.
Mixing with insulins
Due to the lack of compatibility studies, this drug should not be mixed with other drugs, with the exception of NPH human insulin.
Rules for using the SoloStar disposable syringe pen
The manufacturer's instructions for using the SoloStar syringe pen should be studied before the first use of the syringe pen and, if necessary, before each subsequent use.
The patient should be informed about the method of insulin administration.
Syringe pen SoloStar is a disposable syringe pen for insulin injections, with which you can administer a dose from 1 to 80 units at intervals of 1 unit.
Before you start administering insulin, the syringe pen should be inspected. The syringe pen can only be used if the solution in the cartridge is transparent, colorless, does not contain visible mechanical inclusions and has a consistency similar to water. Because the drug is a solution; no pre-mixing is required.
Before first use, it is necessary that the syringe pen is at room temperature for 1-2 hours.
Before injection, air bubbles should be removed from the syringe pen. Empty cartridges are not reused and must be destroyed.
The SoloStar syringe pen is intended for use only on one patient and should not be transferred to another person.
The SoloStar syringe pen should be protected from falls and other external influences, because The cartridge may be damaged, resulting in a malfunction of the syringe pen. If this happens, you should use a new syringe pen.
A new needle should be used before each injection. It is possible to use needles specifically designed for SoloStar or suitable for a syringe pen. After the injection, the needle should be removed and the syringe pen should be stored without a needle. Reuse of the needle is not permitted. The needle should also be removed before disposing of the pen.
To inject insulin using a SoloStar syringe pen, you must consistently follow the steps in paragraphs. I-VI.
I. Checking insulin
1.Before using a pen, you should check the label to ensure you have chosen the correct insulin. The SoloStar syringe pen containing Apidra has a blue body and a dark blue start button.
2. Remove the pen cap.
3. Check the appearance of the insulin. The solution should be transparent and colorless. If the solution in the cartridge is cloudy or contains mechanical inclusions, then the syringe pen cannot be used.
II. Needle installation
1. Remove the outer protective membrane.
2. Attach the needle to the syringe pen, while keeping the needle straight (depending on the type of needle, it is screwed on or put on). Do not hold the needle at an angle when attaching it to the syringe pen. This can damage the rubber membrane and cause insulin to leak, as well as break the needle.
III. Safety test
This test should be performed before each injection.
1. Dial a dose equal to 2 units on the dose selector.
2. Remove the outer protective cap. Do not throw it away, because... You will need it after the injection. Remove the inner protective cap and throw it away, because... it won't be needed anymore.
3. The syringe pen should be held with the needle up.
4. Tap the cartridge holder with your finger so that air bubbles rise up to the needle.
5. Press the trigger button fully to release the dose. It is necessary to ensure that insulin release has occurred. The safety test can be performed several times until insulin release occurs.
If insulin release does not occur even after repeating the safety test, you should check for air bubbles. If air bubbles are present, the safety test should be repeated until they disappear. If there are no air bubbles, the needle may be clogged. In this case, the needle should be replaced.
IV. Setting and dialing insulin dosage
You can set the dose from 1 to 80 units in 1 unit increments. If a dose of more than 80 units is required, it should be administered in two or more injections.
1. Check the value in the dose indicator window after performing the safety test. It should be equal to "0".
2. Dial the required dose. If the wrong dose was dialed, you should turn the dose selector in the opposite direction to the required value.
Do not press the start button while selecting a dose, because Insulin may be released, resulting in incorrect dosing.
The dose selector can only be turned by the number of dose units contained in the pen, so do not turn the dose selector further than is possible. If the pen does not contain enough insulin, you can inject the missing amount using a new pen or inject the entire dose from a new pen.
V. Insulin injection
1. The patient should use the method of administration as prescribed by the physician.
2. Insert the needle into the skin.
3. Press the start button all the way. The dose value in the dose indicator window should return to the value “0”.
4. Without removing the needle and holding the start button, you should count to 10. This is necessary in order to ensure that the dose is completely administered. Remove the needle.
VI. Removing the needle
After injection, you should always disconnect the needle and store the pen without the needle. This will prevent the needle from getting dirty, air bubbles accumulating in the cartridge holder, and insulin leaking.
1. To prevent accidental damage, you should put the outer protective cap on the needle.
2. Disconnect the needle. Dispose of the used needle.
3. Place the cap on the syringe pen.
Buy Apidra SoloStar solution subcutaneously 100 IU/ml 3ml syringe pen No. 5 in pharmacies
Apidra SoloStar Buy Apidra SoloStar in pharmacies DOSAGE FORMS solution for subcutaneous injection 100 U/ml
MANUFACTURERS Sanofi-Aventis Vostok (Russia) Sanofi-Aventis Deutschland GmbH packaged Sanofi-Aventis Vostok (Germany)
GROUP Short-acting insulins
COMPOSITION Active substance: insulin glulisine. Excipients: m-cresol, trometamol, sodium chloride, polysorbate 20, sodium hydroxide, concentrated hydrochloric acid, water for injection.
INTERNATIONAL NON-PROPENTED NAME Insulin glulisine
SYNONYMS Apidra
PHARMACOLOGICAL ACTION Insulin glulisine is a recombinant analogue of human insulin, which is equal in potency to soluble human insulin, but begins to act faster and has a shorter duration of action. The most important action of insulin and insulin analogues, including insulin glulisine, is the regulation of glucose metabolism. Insulin lowers blood glucose concentrations by stimulating glucose uptake into peripheral tissues, especially skeletal muscle and adipose tissue, and also inhibits glucose production in the liver. Insulin suppresses lipolysis in adipocytes, proteolysis and increases protein synthesis. With subcutaneous administration, the hypoglycemic effect develops within 10-20 minutes. When administered intravenously, the hypoglycemic effects of insulin glulisine and soluble human insulin are equal in strength. One unit of insulin glulisine has the same hypoglycemic activity as one unit of soluble human insulin. In insulin glulisine, replacing the amino acid asparagine of human insulin at position B3 with lysine and lysine at position B29 with glutamic acid promotes faster absorption from the injection site. Pharmacokinetic concentration-time curves in healthy volunteers and patients with type 1 and type 2 diabetes mellitus demonstrated that the absorption of insulin glulisine, compared with soluble human insulin, was approximately 2-fold faster, achieving approximately 2-fold greater maximum concentrations.
INDICATIONS FOR USE Diabetes mellitus requiring treatment with insulin (in adults).
CONTRAINDICATIONS Hypoglycemia, hypersensitivity to insulin glulisine or to any of the components of the drug. Should be used with caution during pregnancy. When prescribing the drug during pregnancy, caution should be exercised. Careful monitoring of blood glucose levels is mandatory. Patients with diabetes mellitus (including gestational diabetes) need to maintain optimal metabolic control throughout pregnancy. During breastfeeding, adjustments in insulin doses and diet may be required.
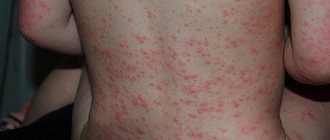
SIDE EFFECTS Hypoglycemia is the most common undesirable effect of insulin therapy, which can occur when doses of insulin are used that are too high, exceeding the need for it. From the side of metabolism: very often - hypoglycemia. Symptoms of hypoglycemia usually occur suddenly. These include cold sweats, pale skin, feeling tired, nervousness or tremors, restlessness, weakness, confusion, difficulty concentrating, drowsiness, excessive hunger, visual disturbances, headache, nausea and palpitations. Hypoglycemia may worsen, leading to loss of consciousness and/or seizures, temporary or permanent impairment of brain function, or even death. Local reactions: often - local hypersensitivity reactions (hyperemia, swelling and itching at the injection site). These reactions are usually transient and disappear with continued treatment. Rarely - lipodystrophy (as a result of a violation of the alternation of insulin injection sites in any of the areas /administration of the drug in the same place/). Allergic reactions: sometimes - urticaria, chest tightness, bronchospasm, allergic dermatitis, itching. Severe cases of generalized allergic reactions (including anaphylactic reactions) can be life-threatening.
INTERACTION When used together, oral hypoglycemic agents, ACE inhibitors, disopyramide, fibrates, fluoxetine, MAO inhibitors, pentoxifylline, propoxyphene, salicylates and sulfonamide antimicrobial agents may enhance the hypoglycemic effect of insulin and increase susceptibility to hypoglycemia. When used together, GCS, danazol, diazoxide, diuretics, isoniazid, phenothiazine derivatives, somatropin, sympathomimetics (for example, epinephrine / adrenaline /, salbutamol, terbutaline), thyroid hormones, estrogens, progestins (for example, oral contraceptives), protease inhibitors and antipsychotics drugs (eg, olanzapine and clozapine) may reduce the hypoglycemic effect of insulin. Beta blockers, clonidine, lithium salts or ethanol can either potentiate or weaken the hypoglycemic effect of insulin. Pentamidine can cause hypoglycemia followed by hyperglycemia. When using drugs with sympatholytic activity (beta-blockers, clonidine, guanethidine and reserpine), symptoms of reflex adrenergic activation during hypoglycemia may be less pronounced or absent. Due to the lack of compatibility studies, insulin glulisine should not be mixed with any other drugs except human isophane insulin. When administered using an infusion pump, Apidra should not be mixed with other drugs.
OVERDOSE Symptoms: there are no specific data on overdose of insulin glulisine; Hypoglycemia of varying severity may develop. Treatment: Episodes of mild hypoglycemia can be treated with glucose or sugar-containing foods. Therefore, it is recommended that patients with diabetes have sugar cubes, candies, cookies or sweet fruit juice with them at all times. Episodes of severe hypoglycemia, during which the patient loses consciousness, can be stopped by intramuscular or subcutaneous administration of 0.5-1 mg of glucagon or intravenous administration of dextrose (glucose). If the patient does not respond to glucagon administration within 10-15 minutes, It is also necessary to administer dextrose intravenously. After regaining consciousness, it is recommended to give the patient carbohydrates orally to prevent recurrence of hypoglycemia. After administration of glucagon, the patient should be observed in the hospital to determine the cause of this severe hypoglycemia and prevent the development of other similar episodes.
SPECIAL INSTRUCTIONS Transfer of a patient to a new type of insulin or insulin from another manufacturer should be carried out under strict medical supervision, because All therapy may need to be adjusted. Using inadequate doses of insulin or stopping treatment, especially in patients with type 1 diabetes, can lead to the development of hyperglycemia and diabetic ketoacidosis, conditions that are potentially life-threatening. Adjustment of insulin doses may also be required when changing physical activity or eating patterns. Physical activity performed immediately after a meal may increase the risk of hypoglycemia. Compared with soluble human insulin, hypoglycemia may develop earlier after injection of rapid-acting insulin analogues. Uncompensated hypoglycemic or hyperglycemic reactions can lead to loss of consciousness, coma or death. The need for insulin may change with concomitant diseases or emotional overload.
STORAGE CONDITIONS OptiClick cartridges and cartridge systems should be stored out of the reach of children, protected from light at a temperature of 2° to 8°C, and not frozen. After starting to use, OptiClick cartridges and cartridge systems should be stored out of the reach of children, protected from light at a temperature not exceeding 25°C. To protect from light exposure, cartridges and OptiClick cartridge systems should be stored in their own cardboard packaging. It is recommended to mark the date of first collection of the drug on the label.
Apidra® SoloStar®
Transferring a patient to a new type of insulin or insulin from another manufacturer should be carried out under strict medical supervision, since a change in dosage may be required due to changes in insulin concentration, brand (manufacturer), type of insulin (soluble, isophane insulin, etc.), type insulin (animal origin) and/or production method. In addition, adjustment of concomitant oral hypoglycemic therapy may be required. Using inadequate doses of insulin or stopping treatment, especially in patients with type 1 diabetes, can lead to the development of hyperglycemia and diabetic ketoacidosis, conditions that are potentially life-threatening.
Hypoglycemia
The time it takes for hypoglycemia to develop depends on the speed of onset of the effect of the insulins used and, therefore, may change when the treatment regimen changes. Conditions that may alter or lessen the warning signs of hypoglycemia include: prolonged diabetes mellitus, intensification of insulin therapy, the presence of diabetic neuropathy, taking certain medications such as beta-blockers, or switching the patient from animal-derived insulin to human insulin.
Insulin dosage adjustments may also be necessary if patients increase physical activity or change their usual eating routine. Physical activity performed immediately after a meal may increase the risk of hypoglycemia. Compared with soluble human insulin, hypoglycemia may occur earlier after injection of fast-acting insulin analogues.
Uncompensated hypoglycemic or hyperglycemic reactions can lead to loss of consciousness, coma or death.
The need for insulin may change due to illness or emotional overload.
SoloStar®
syringe pen Before first use, the syringe pen must be kept at room temperature for 1-2 hours.
Before use, you should inspect the cartridge inside the syringe pen. It should only be used if the solution is clear, colorless, contains no visible solids, and has a consistency similar to water.
Empty SoloStar® syringe pens should not be reused and must be destroyed.
To prevent infection, the prefilled pen should only be used by one patient and not shared with another person.
Handling the SoloStar® syringe pen
Before using the SoloStar® syringe pen, carefully read the information on use.
Important information on
SoloStar®
syringe pen Before each use, you must carefully connect a new needle to the syringe pen and conduct a safety test. It is necessary to use only needles that are compatible with the SoloStar® syringe pen.
Special precautions must be taken to avoid needle-related accidents and the possibility of transmitting infection.
Do not use the SoloStar® syringe pen under any circumstances if it is damaged or if you are not sure that it will work properly.
Always have a spare SoloStar® syringe pen available in case your copy of the SoloStar® syringe pen is lost or damaged.
Storage instructions
Please study the “Storage Conditions” section regarding the rules for storing the SoloStar® syringe pen.
If the SoloStar® syringe pen is stored in the refrigerator, remove it from there 1-2 hours before the intended injection so that the solution reaches room temperature. Injecting chilled insulin is more painful.
The used SoloStar® syringe pen must be destroyed.
Caring for the SoloStar syringe pen
®
The SoloStar® syringe pen must be protected from dust and dirt. The outside of the SoloStar® syringe pen can be cleaned by wiping it with a damp cloth.
Do not immerse, rinse or lubricate the SoloStar® syringe pen in liquid, as this may damage it.
The SoloStar® syringe pen accurately doses insulin and is safe to use. It also requires careful handling. Avoid situations in which damage to the SoloStar® syringe pen may occur. If you suspect that your SoloStar® syringe pen may have been damaged, use a new syringe pen.
Stage 1: Insulin control
It is necessary to check the label on the SoloStar® pen to ensure that it contains the appropriate insulin. For the drug Apidra®, the SoloStar® syringe pen is blue with a dark blue button with a relief ring for administering the injection. After removing the cap of the syringe pen, the appearance of the insulin contained in it is controlled: the insulin solution should be transparent, colorless, free of visible solid particles and resemble water in consistency.
Stage 2. Connecting the needle
It is necessary to use only needles that are compatible with the SoloStar® syringe pen.
For each subsequent injection, always use a new sterile needle. After removing the cap, the needle must be carefully installed on the syringe pen.
Stage 3
.
Performing a safety test
Before each injection, you should perform a safety test to ensure that the pen and needle are working properly and that any air bubbles have been removed.
Measure out a dose equal to 2 units. The outer and inner needle caps must be removed.
With the syringe pen facing upward, gently tap the insulin cartridge with your finger so that all air bubbles are directed towards the needle. Fully press the injection button.
If insulin appears at the tip of the needle, the pen and needle are working correctly.
If no insulin appears at the needle tip, then step 3 can be repeated until insulin appears at the needle tip.
Stage 4. Dose selection
The dose can be set to within 1 unit from a minimum dose of 1 unit to a maximum dose of 80 units. If it is necessary to administer a dose greater than 80 units, 2 or more injections should be given. The dosage window should show “0” after completion of the safety test. After this, the required dose can be set.
Stage 5. Dose administration
The patient should be informed about the injection technique by a healthcare professional.
The needle must be inserted under the skin. The injection button must be pressed fully. It is held in this position for another 10 s until the needle is removed. This ensures that the selected dose of insulin is administered completely.
Stage 6: Removing and discarding the needle
In all cases, the needle should be removed and discarded after each injection. This prevents contamination and/or infection, air from entering the insulin container, and insulin leakage.
Special precautions must be taken when removing and discarding the needle. Follow recommended safety precautions for removing and disposing of needles (eg, one-handed capping technique) to reduce the risk of needle-related accidents and prevent infection.
After removing the needle, you should close the SoloStar® syringe pen with the cap.