Buy Octreotide solution intravenously and subcutaneously 0.01% 1ml No. 10 in pharmacies
Octreotide Buy Octreotide in pharmacies Octreotide in the drug directory DOSAGE FORMS solution for intravenous and subcutaneous administration 50 µg/ml solution for injection 0.01%
MANUFACTURERS Deco Company (Russia) Pharm-Sintez (Russia)
GROUP Somatotropin antagonists
COMPOSITION The active substance is octreotide.
INTERNATIONAL NON-PROPENTED NAME Octreotide
SYNONYMS Genfastat, Octreotide FSintez, Octreotide-depot, Octreotide-long FS, Octride, Sandostatin, Sandostatin Lar, Seraxtal
PHARMACOLOGICAL ACTION Pharmacological action is somatostatin-like. Inhibits the production of growth hormone, reduces the secretion of glucagon, insulin, serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin and pancreatic polypeptide. Reduces blood flow in visceral organs. Eliminates symptoms associated with metastases of carcinoid tumors (hot flashes and diarrhea), increased secretion (adenomas) of vasoactive intestinal peptide (diarrhea), glucagon (diarrhea, weight loss, necrotizing migratory rash), insulin (hypoglycemia). Significantly reduces the concentration of growth hormone and/or somatomedin C in patients with acromegaly, the production of thyrotropin stimulated by thyrotropin-releasing hormone. Inhibits the contractility of the gallbladder, suppresses the flow of bile into the duodenum. After subcutaneous administration, it is quickly and completely absorbed. The maximum concentration is achieved within 25-30 minutes. The half-life after injection is 100 minutes. The duration of action is variable, on average about 12 hours, depending on the type of tumor. About 32% of the administered dose is excreted unchanged in the urine. In elderly patients, the clearance of octreotide is reduced and the half-life is prolonged. In severe renal failure requiring hemodialysis, clearance is reduced by half.
INDICATIONS FOR USE Acromegaly (if dopamine agonists are ineffective or surgical treatment or radiation therapy is impossible), endocrine tumors of the gastroenteropancreatic system (relief of symptoms of carcinoid tumors with signs of carcinoid syndrome, tumors characterized by overproduction of vasoactive intestinal peptide), glucagonomas, gastrinomas (Zollinger-Ellison syndrome ), insulinomas, somatoliberinomas, refractory diarrhea in patients with AIDS, operations on the pancreas (prevention of complications), bleeding (including prevention of relapses) with varicose veins of the esophagus in patients with cirrhosis of the liver.
CONTRAINDICATIONS Hypersensitivity, pregnancy and breastfeeding (stop during treatment).
SIDE EFFECTS Nausea, vomiting, anorexia, cramping abdominal pain, flatulence, diarrhea and steatorrhea, symptoms of acute intestinal obstruction, liver dysfunction, formation of gallstones (with long-term use), acute pancreatitis, hyper- or hypoglycemia, hair loss; locally - pain, itching or burning sensation, redness, swelling.
INTERACTIONS Reduces serum levels of cyclosporine and slows down the absorption of cimetidine and nutrients from the gastrointestinal tract. Dose adjustment of concomitantly used insulin, oral hypoglycemic drugs, beta-blockers, calcium channel blockers and diuretics is required.
METHOD OF APPLICATION AND DOSAGE Subcutaneously, intravenously (infusion). For acromegaly and tumors of the gastroenteropancreatic system, subcutaneously - 50-100 mcg 1-2 times a day (up to 100-200 mcg 3 times a day); For refractory diarrhea in AIDS, subcutaneously - 100 mcg 3 times a day (up to 250 mcg 3 times a day). In order to prevent complications after pancreatic surgery, the first dose of 100 mcg is administered subcutaneously 1 hour before laparotomy, then after surgery, 100 mcg is administered subcutaneously 3 times a day for seven consecutive days. To stop bleeding from varicose veins of the esophagus or stomach - 25 mcg/hour by continuous intravenous infusion for 5 days.
OVERDOSE Symptoms: bradycardia, flushing, diarrhea, nausea, feeling of emptiness and/or pain in the stomach. Treatment: symptomatic.
SPECIAL INSTRUCTIONS Significant fluctuations in blood glucose concentrations can be reduced by more frequent administration of smaller doses; It should be borne in mind that when treating gastroenteropancreatic endocrine tumors, a sudden relapse of symptoms cannot be excluded, and in patients with insulinomas, an increase in the severity and duration of hypoglycemia. Systematic monitoring of glucose concentration is necessary, especially in patients with bleeding from esophageal varices with cirrhosis of the liver, because the risk of developing type 1 diabetes mellitus (insulin-dependent) increases; the need for insulin changes if you already have diabetes.
STORAGE CONDITIONS List B. At a temperature of 2 to 8 degrees. C, in a place protected from light.
Pharmacological properties of the drug Octreotide
A synthetic octapeptide, which is a derivative of the natural hormone somatostatin and has pharmacological effects similar to it, but a much longer duration of action. Suppresses pathologically increased secretion of growth hormone, as well as peptides and serotonin produced in the gastroenteropancreatic zone. In acromegaly, octreotide reduces the concentration of growth hormone and/or somatomedin C in the blood plasma and the severity of symptoms such as headache, swelling, hyperhidrosis, joint pain and paresthesia. In endocrine tumors of the digestive tract and pancreas, octreotide changes some clinical manifestations of the disease. For tumors characterized by overproduction of vasoactive intestinal peptide (VIP), the use of octreotide in most patients leads to a decrease in the severity of secretory diarrhea. At the same time, there is a reduction in accompanying electrolyte imbalances. For glucagonomas, the use of the drug in most cases leads to a noticeable reduction in the necrotizing migratory rash that is characteristic of this condition. Octreotide does not have any significant effect on the severity of diabetes mellitus, often observed in glucagonomas, and usually does not lead to a reduction in the need for insulin or oral hypoglycemic drugs. The decrease in diarrhea observed during therapy is accompanied by an increase in the patient’s body weight. At the beginning of treatment, a rapid decrease in the concentration of glucagon in the blood plasma is often observed, but with long-term therapy this effect does not persist. At the same time, symptomatic improvement remains stable over a long period of time. For gastrinomas (Zollinger-Ellison syndrome), octreotide, used as monotherapy or in combination with H2-receptor blockers, can reduce acid production in the stomach, reduce the severity of diarrhea, hot flashes and other symptoms likely associated with the synthesis of peptides by the tumor. In some cases, there is a decrease in the concentration of gastrin in the blood plasma. In patients with insulinomas, octreotide reduces the level of immunoreactive insulin in the blood (this effect, however, may be short-lived - about 2 hours). In patients with a resectable tumor, octreotide can restore and maintain normoglycemia in the preoperative period. In patients with inoperable benign and malignant tumors, glycemic control may improve without a simultaneous prolonged decrease in blood insulin levels. In patients with a tumor that produces growth hormone RF (somatoliberinomas), octreotide reduces the severity of symptoms of acromegaly. In the future, pituitary hypertrophy may decrease. For refractory diarrhea in AIDS patients, the use of octreotide leads to complete or partial normalization of stool in approximately 1/3 of patients. The use of octreotide during and after pancreatic surgery reduces the incidence of typical postoperative complications (for example, pancreatic fistulas, abscesses, sepsis, postoperative acute pancreatitis). In the case of bleeding from varices of the esophagus and stomach in patients with cirrhosis of the liver, the use of octreotide in combination with specific treatment (for example, sclerotherapy) leads to more effective control of bleeding and prevention of early rebleeding, a reduction in the volume of transfusions and an improvement in 5-day survival, probably , by suppressing the production of vasoactive hormones such as vasoactive intestinal peptide and glucagon.
Results and discussion
Already at the stage of univariate analysis, i.e., with a simple comparison of groups, a significantly lower mortality rate was revealed in the group of patients receiving octreotide compared with the group that did not receive it (52% versus 72%; χ2 test; p
=0.003).
When comparing groups by the prevalence of necrosis of the pancreas and parapancreatic tissue, it was also found that in the main group, widespread necrosis was significantly less common than in the control group (64% versus 77%; χ2 test; p
= 0.051). Thus, evaluation of the effectiveness of octreotide by univariate statistical analysis, used only in patients with a prognostically severe course of the disease, and not on the total number of patients with pancreatitis, as in the study by W. Uhl et al., showed a decrease in mortality and the prevalence of necrosis.
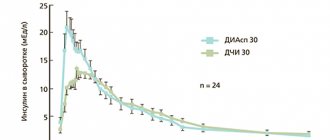
To adequately interpret this result and exclude the possibility of indirect correlation, the groups were compared for other potential predictors, which could also influence mortality and the prevalence of necrosis. Analysis of the severity of the condition of patients in both groups upon admission revealed virtually no differences between them (Table 1). The integral index of organ dysfunction (according to the APACHE II scale) did not differ in both groups (12.0±3.3 points in the main group versus 12.0±3.3 points in the control group; t
-test;
p
=0.959) (see figure).
Table 1. Differences in factors of severity of the condition of patients in the main and control groups
The severity of the patient's condition upon admission in patients with an initially severe course of the disease.
However, differences in treatment factors were significant. To exclude the fact that the detected decrease in mortality and prevalence of necrosis in the group of patients receiving octreotide was due to more frequent use of nutritional support at an early stage of the disease (χ2 test; p
=0.000) and pancreatic antibiotics (
U
-test;
p
=0.000) (Table 2), rather than using octreotide, turned out to be impossible. In this regard, to establish the cumulative effect of octreotide on mortality with other factors, a method of statistical analysis was used - the Cox proportional hazards model.
Table 2. Differences in treatment factors in the main and control groups
Cox analysis (Table 3) made it possible to construct a significant model of treatment factors and severity of the condition affecting mortality ( p
=0.000).
Octreotide was included in the predictor model ( p
= 0.000).
Table 3. Cox regression results
Moreover, the regression coefficient for octreotide turned out to be the largest of all predictors included in the model (β = –0.8). This meant that the strength of its influence on changes in mortality was the greatest among all factors included in the model. The sign of the regression coefficient “–” indicated that the fact of completeness of observation of the patient’s lifespan (corresponding to the fact of death) and the prescription of octreotide are inversely related, i.e., the prescription of octreotide reduced the likelihood of death in patients with destructive pancreatitis.
To determine the effect of octreotide administration on the prevalence of necrosis of the pancreas and parapancreatic tissue, logistic regression analysis was used (Table 4), which made it possible to construct a significant model of the mutual influence of factors ( p
=0.0001).
This model, along with other predictors that mutually influence the development of widespread necrosis, included octreotide ( p
= 0.033) as the factor with the strongest influence, the regression coefficient was –0.875. The sign of the regression coefficient “–” indicated that the change in the prevalence of pancreatic necrosis and the administration of octreotide are inversely related, i.e., the administration of octreotide reduced the likelihood of the prevalence of necrosis in patients with destructive pancreatitis.
Table 4. Results of logistic regression on the prevalence of necrosis
Thus, using the Cox proportional hazards model, a pattern was identified that the use of octreotide has a very strong effect on reducing mortality in initially severe forms of pancreatic necrosis. Using logistic regression, it was found that octreotide is the most significant factor influencing the reduction in the prevalence of necrosis of the pancreas and parapancreatic tissue in patients with severe pancreatic necrosis.
The data from the study allow us to recommend the administration of octreotide to patients with initially severe pancreatic necrosis.
Use of the drug Octreotide
For acromegaly, octreotide is first administered at 0.05–0.1 mg subcutaneously at intervals of 8 or 12 hours. Subsequently, the dose is selected individually. Typically, the optimal daily dose is 0.2–0.3 mg. The maximum dose of 1.5 mg per day should not be exceeded. If after 3 months of treatment there is no sufficient reduction in growth hormone levels and improvement in the clinical picture of the disease, therapy should be discontinued. For endocrine tumors of the digestive tract and pancreas, octreotide is administered subcutaneously at an initial dose of 0.05 mg 1–2 times a day. Subsequently, depending on the achieved clinical effect, the effect on the levels of hormones produced by the tumor (in the case of carcinoid tumors, the effect on the excretion of 5-hydroxyindoleacetic acid in the urine), and tolerability, the dose of octreotide can be gradually increased to 0.1–0.2 mg 3 once a day. In exceptional cases, higher doses may be required. Maintenance doses are selected individually. For refractory diarrhea in patients with AIDS, octreotide is administered subcutaneously at an initial dose of 0.1 mg 3 times a day. If after 1 week of treatment the symptoms of diarrhea do not disappear, the dose should be increased individually up to 0.25 mg 3 times a day. Dose adjustment is carried out taking into account the dynamics of bowel movements and tolerability of the drug. If no improvement occurs within 1 week of treatment with octreotide at a dose of 0.25 mg 3 times daily, therapy should be discontinued. To prevent complications after pancreatic surgery, 0.1 mg is administered subcutaneously 3 times a day for 7 consecutive days starting from the day of surgery (at least 1 hour before laparotomy). For bleeding from varicose veins of the esophagus, a dose of 25 mcg/hour is administered by continuous intravenous infusion for 5 days.
Indications for use of the drug Octreotide
Acromegaly (control of the main manifestations of the disease and a decrease in the level of growth hormone and somatomedin C in the blood plasma in cases where the effect of surgical treatment, radiation therapy and treatment with dopamine agonists is not sufficient); relief of symptoms of endocrine tumors of the digestive tract and pancreas: carcinoid tumors with the presence of carcinoid syndrome; VIPs; glucagonomas; gastrinomas/Zollinger-Ellison syndrome (usually in combination with H2-histamine receptor blockers); insulinomas (to control hypoglycemia in the preoperative period, as well as for maintenance therapy); somatoliberins; refractory diarrhea in patients with AIDS; prevention of complications after pancreatic surgery; stopping bleeding and preventing re-bleeding from esophageal varices in patients with cirrhosis of the liver (in combination with specific therapeutic measures, for example, endoscopic sclerotherapy).
Side effects of the drug Octreotide
Possible pain, itching or burning sensation, redness and swelling at the injection site, anorexia, nausea, vomiting, cramping abdominal pain, bloating, flatulence, loose stools, diarrhea and steatorrhea, phenomena resembling acute intestinal obstruction (progressive bloating, severe pain in the epigastric region, muscle protection), formation of gallstones (with long-term use in 10–20% of patients), acute pancreatitis, hair loss, liver dysfunction, including acute hepatitis without cholestasis, hyperbilirubinemia, accompanied by increased alkaline levels phosphatases, γ-glutamyltransferases and, to a lesser extent, transaminases, decreased glucose tolerance, persistent hyperglycemia or hypoglycemia (with long-term use).
Special instructions for the use of the drug Octreotide
In case of a pituitary tumor that secretes growth hormone, strict medical supervision of patients receiving octreoid is necessary, since it is possible that the size of the tumors may increase with the development of such a serious complication as a narrowing of the visual fields. When treating endocrine tumors of the digestive tract and pancreas with octreotide, in rare cases a sudden relapse of the disease may occur. In patients with insulinoma during treatment with octreoid, an increase in the severity and duration of hypoglycemia may be observed. In diabetic patients receiving insulin, Octreoid may reduce the need for insulin. There is no experience with the use of Octreoid during pregnancy and lactation; during this period the drug is prescribed only for absolute indications.