Rp.: Sol. A drenalini hydrochloridi
0.1% – 1 ml
DtdN 6 in ampullis.
S. SC 0.5 ml
Gr.: -,-adrenergic agonist.
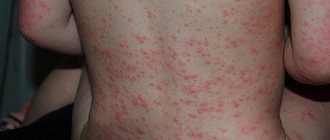
Pok.: for anaphylactic shock and allergic edema of the larynx, attack of bronchial asthma, hypotonic conditions, for prolonging the action of local anesthetics, for insulin overdose, for pupil dilation (without impaired accommodation and increased intraocular pressure), for cardiac arrest (intracardiac)
MD, FS: increases blood pressure due to activation of 1-AR of the vessels of the abdominal cavity and extrasynaptic 2-AR, increased heart rate and increased contractility of the heart due to the excitation of 1-AR in it, dilates the vessels of skeletal muscles due to the excitation of 2 in them -AR, dilates the bronchi and increases mucin production, activating 2-AR, activates hepatic glycogenesis.
Aminazine
Rp.: Sol. Aminazini
2.5% – 1 ml
DtdN 6 in ampullis.
S. For intramuscular injections; dilute in 5 ml of 0.5% novocaine solution.
Gr.: neuroleptic.
Pok.: for the treatment of schizophrenia, psychoses, for potentiating the action of anesthesia, hypnotics and painkillers, for absthenic syndrome in alcoholics, as an antiemetic and antihiccal agent, for creating artificial hypothermia, for relieving hypertensive crises, for trigeminal neuralgia.
MD, FS: penetrating the brain, blocks dopamine, adrenergic, serotonergic, cholinergic receptors. Reduces psychomotor agitation, motor activity, aggressiveness, inhibits conditioned reflexes, has an antipsychotonic effect, has an α-AB effect (dilates blood vessels, reduces blood pressure), has antiemetic and antihiccup activity.
Adrenaline hydrotartrate 0.18%/1 ml No. 10 solution d/in.amp.
Instructions for medical use of the drug ADRENALIN-HEALTH Trade name Adrenaline-Zdorovye International nonproprietary name Epinephrine Dosage form Solution for injection 0.18%, 1 ml Composition 1 ml of solution contains the active substance - adrenaline hydrotartrate 1.82 mg excipients: sodium metabisulfite ( E 223), sodium chloride, water for injection Description Transparent colorless solution Pharmacotherapeutic group Drugs for the treatment of heart diseases. Cardiotonic drugs of non-glycoside origin. Adrenergic and dopamine stimulants. Epinephrine. ATX code C01CA24. Pharmacological properties Pharmacokinetics After intramuscular or subcutaneous administration, epinephrine is rapidly absorbed; the maximum concentration in the blood is reached after 3-10 minutes. The therapeutic effect develops almost instantly with intravenous administration (duration of action is 1-2 minutes), 5-10 minutes after subcutaneous administration (maximum effect after 20 minutes), with intramuscular administration the onset of the effect is variable. Penetrates through the placental barrier, into breast milk, does not penetrate the blood-brain barrier. Metabolized by monoamine oxidase (to vanillylmandelic acid) and catechol-O-methyltransferase (to metanephrine) in the cells of the liver, kidneys, intestinal mucosa, and axons. The half-life when administered intravenously is 1-2 minutes. Excretion of metabolites is carried out by the kidneys. Excreted in breast milk. Pharmacodynamics Adrenaline-Health is a cardiostimulating, vasoconstrictor, hypertensive, antihypoglycemic agent. Stimulates α- and β-adrenergic receptors of various localizations. It has a pronounced effect on the smooth muscles of internal organs, the cardiovascular and respiratory systems, and activates carbohydrate and lipid metabolism. The mechanism of action is due to the activation of adenylate cyclase on the inner surface of cell membranes, an increase in the intracellular concentration of cAMP and Ca2+. The first phase of action is primarily due to stimulation of β-adrenergic receptors in various organs and is manifested by tachycardia, increased cardiac output, myocardial excitability and conductivity, arteriolo- and bronchodilation, decreased uterine tone, mobilization of glycogen from the liver and fatty acids from fat depots. In the second phase, α-adrenergic receptors are excited, which leads to vasoconstriction of the abdominal organs, skin, mucous membranes (skeletal muscles to a lesser extent), an increase in blood pressure (mainly systolic), and general peripheral vascular resistance. The effectiveness of the drug depends on the dose. In very low doses, at a rate of administration less than 0.01 mcg/kg/min, it can reduce blood pressure due to vasodilation of skeletal muscles. At an injection rate of 0.04-0.1 mcg/kg/min, it increases the frequency and strength of heart contractions, stroke volume and minute blood volume, and reduces total peripheral vascular resistance; above 0.2 mcg/kg/min – constricts blood vessels, increases blood pressure (mainly systolic) and total peripheral vascular resistance. The pressor effect can cause a short-term reflex slowing of the heart rate. Relaxes the smooth muscles of the bronchi. Doses above 0.3 mcg/kg/min reduce renal blood flow, blood supply to internal organs, tone and motility of the gastrointestinal tract. Increases conductivity, excitability and automatism of the myocardium. Increases myocardial oxygen demand. Inhibits the release of histamine and leukotrienes induced by antigens, eliminates spasm of bronchioles, and prevents the development of edema of their mucous membrane. Acting on α-adrenergic receptors of the skin, mucous membranes and internal organs, it causes vasoconstriction, reduces the rate of absorption of local anesthetics, increases the duration of action and reduces the toxic effect of local anesthesia. Stimulation of β2-adrenergic receptors is accompanied by increased excretion of potassium from the cell and can lead to hypokalemia. When administered intracavernosally, it reduces the blood supply to the cavernous bodies. Dilates the pupils, helps reduce the production of intraocular fluid and intraocular pressure. Causes hyperglycemia (increases glycogenolysis and gluconeogenesis) and increases the content of free fatty acids in the blood plasma, improves tissue metabolism. It weakly stimulates the central nervous system and exhibits antiallergic and anti-inflammatory effects. Indications for use - allergic reactions of immediate type: anaphylactic shock that developed during the use of drugs, serums, blood transfusions, insect bites or contact with allergens - relief of acute attacks of bronchial asthma - arterial hypotension of various origins (post-hemorrhagic, intoxication, infectious) - hypokalemia, including due to an overdose of insulin - asystole, cardiac arrest - prolongation of the action of local anesthetics - AV block of the third degree, acutely developed Method of administration and dosage Prescribed intramuscularly, subcutaneously, intravenously (drip), intracardially (resuscitation in case of cardiac arrest). When administered intramuscularly, the effect of the drug develops faster than when administered subcutaneously. The dosage regimen is individual. Adults. Anaphylactic shock: 0.5 ml, diluted in 20 ml of 40% glucose solution, is administered intravenously slowly. Subsequently, if necessary, intravenous drip administration is continued at a rate of 1 mcg/min, for which 1 ml of adrenaline solution is dissolved in 400 ml of isotonic sodium chloride or 5% glucose. If the patient's condition allows it, it is better to administer intramuscular or subcutaneous injection of 0.3-0.5 ml in a diluted or undiluted form. Bronchial asthma: 0.3-0.5 ml is administered subcutaneously, diluted or undiluted. If repeated administration is necessary, this dose can be administered every 20 minutes (up to 3 times). Intravenous administration of 0.3-0.5 ml in diluted form is possible. As a vasoconstrictor, it is administered intravenously at a rate of 1 mcg/min (with a possible increase to 2-10 mcg/min). Asystole: 0.5 ml diluted in 10 ml of 0.9% sodium chloride solution is administered intracardially. During resuscitation measures - 1 ml (diluted) intravenously slowly every 3-5 minutes. Children. Asystole in newborns: administered intravenously at 0.01 ml/kg body weight every 3-5 minutes, slowly. Anaphylactic shock: administered subcutaneously or intramuscularly to children under 1 year of age - 0.05 ml, at the age of 1 year - 0.1 ml, 2 years - 0.2 ml, 3-4 years - 0.3 ml, 5 years – 0.4 ml, 6-12 years – 0.5 ml. If necessary, administration is repeated every 15 minutes (up to 3 times). Bronchospasm: 0.01 ml/kg body weight is administered subcutaneously (maximum up to 0.3 ml). If necessary, administration is repeated every 15 minutes (up to 3-4 times) or every 4 hours. Side effects Often: - headache - anxiety - tremor - nausea, vomiting - anorexia - hyperglycemia Uncommon: - angina, bradycardia or tachycardia, palpitations, decrease or increase in blood pressure (even with subcutaneous administration in normal doses, an increase in blood pressure is possible subarachnoid hemorrhage and hemiplegia) - shortness of breath - nervousness, dizziness, fatigue, sleep disturbance - muscle twitching - psychoneurotic disorders (psychomotor agitation, disorientation) - memory impairment - aggressive or panic behavior - schizophrenia-like disorders, paranoia - increased rigidity and tremor (in patients with Parkinson's disease) - angioedema, bronchospasm - skin rash, erythema multiforme - increased sweating, impaired thermoregulation, cold extremities Rarely: - ventricular arrhythmias, chest pain - ECG changes (including decreased T wave amplitude) - difficult and painful urination (with prostatic hyperplasia) - hypokalemia - pulmonary edema - pain or burning at the site of intramuscular injection; with repeated injections of adrenaline, necrosis may occur due to the vasoconstrictor effect of adrenaline Contraindications - increased individual sensitivity to the components of the drug - hypertrophic obstructive cardiomyopathy - severe aortic stenosis - tachyarrhythmia, ventricular fibrillation - arterial or pulmonary hypertension - ischemic pulmonary disease - severe atherosclerosis - occlusive vascular diseases - pheochromocytoma - closed-angle glaucoma - shock of non-allergenic origin - convulsive syndrome - thyrotoxicosis - diabetes mellitus - general anesthesia with the use of inhalation agents: fluorothane, cyclopropane, chloroform - pregnancy and lactation, second stage of labor - use on the fingers and toes, on the nose, genitals Drug interactions Epinephrine antagonists are α- and β-adrenergic receptor blockers. With the simultaneous use of the drug Adrenaline-Zdorovye with other drugs, it is possible: - with narcotic analgesics and hypnotics - weakening of their effects; - with cardiac glycosides, quinidine, tricyclic antidepressants, dopamine, inhalation anesthetics (chloroform, enflurane, halothane, isoflurane, methoxyflurane), cocaine - increased risk of arrhythmias; - with other sympathomimetic drugs - increased severity of side effects from the cardiovascular system; - with antihypertensive drugs (including diuretics) - decrease in their effectiveness; - with monoamine oxidase inhibitors (including furazolidone, procarbazine, selegiline) - sudden and severe increase in blood pressure, hyperpyretic crisis, headache, cardiac arrhythmias, vomiting; - with nitrates - weakening of their therapeutic effect; - with phenoxybenzamine - increased hypotensive effect and tachycardia; - with phenytoin - a sudden decrease in blood pressure and bradycardia, depending on the dose and rate of administration of adrenaline; - with thyroid hormone preparations - mutual enhancement of action; − with astemizole, cisapride, terfenadine – prolongation of the QT interval on the ECG; - with diatrizoatams, iothalamic or ioxaglic acids - increased neurological effects; - with ergot alkaloids - increased vasoconstrictor effect up to severe ischemia and the development of gangrene; - with hypoglycemic drugs (including insulin) - decreased hypoglycemic effect; - with non-depolarizing muscle relaxants - a decrease in the muscle relaxant effect is possible; - with hormonal contraceptives - may reduce effectiveness. Special instructions Intracardiac administration for asystole, if other methods of eliminating it are not available, and there is an increased risk of developing cardiac tamponade and pneumothorax. If infusion is necessary, a device with a measuring device should be used to regulate the rate of infusion. The infusion should be carried out into a large, preferably central, vein. When carrying out an infusion, it is recommended to monitor the concentration of potassium in the blood serum, blood pressure, diuresis, ECG, central venous pressure, and pulmonary artery pressure. The use of the drug in patients with diabetes mellitus increases glycemia, which requires higher doses of insulin or sulfonylurea derivatives. It is undesirable to use adrenaline for a long time, since narrowing of peripheral vessels can lead to the development of necrosis or gangrene. When discontinuing treatment, the dose of epinephrine should be reduced gradually, since sudden discontinuation of therapy can lead to severe hypotension. Prescribe with caution to patients with ventricular arrhythmia, coronary heart disease, atrial fibrillation, arterial hypertension, pulmonary hypertension, myocardial infarction (if it becomes necessary to use the drug during myocardial infarction, it should be remembered that adrenaline can increase ischemia by increasing myocardial oxygen demand) , metabolic acidosis, hypercapnia, hypoxia, hypovolemia, thyrotoxicosis, in patients with occlusive vascular diseases (arterial embolism, atherosclerosis, Buerger's disease, cold injury, diabetic endarteritis, Raynaud's disease, with cerebral atherosclerosis, Parkinson's disease, with convulsive syndrome, with prostatic hypertrophy glands. In case of hypovolemia, before using sympathomimetics, it is necessary to adequately hydrate patients. Use in pediatrics. Particular care should be taken when administering the drug to children (dosing varies). Recommendations on dosing of the drug for children are given in the section "Dosage and Administration". Use during pregnancy and lactation Do not use during childbirth to correct hypotension, since the drug can delay the second stage of labor by relaxing the muscles of the uterus. When administered in large doses to weaken uterine contractions, it can cause prolonged uterine atony with bleeding. If it is necessary to use the drug, you should stop breastfeeding, as there is a high probability of side effects in the child. Features of the effect of the drug on the ability to drive vehicles and operate complex mechanisms. During treatment with the drug, it is not recommended to drive vehicles or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions. Overdose Symptoms: excessive increase in blood pressure, mydriasis, tachyarrhythmia followed by bradycardia, cardiac arrhythmia (including atrial and ventricular fibrillation), coldness and pallor of the skin, vomiting, fear, anxiety, tremor, headache, metabolic acidosis, myocardial infarction , cranial hemorrhage (especially in elderly patients), pulmonary edema, renal failure. Treatment: discontinuation of drug administration. Symptomatic therapy, the use of α- and β-blockers, fast-acting nitrates. For arrhythmia, parenteral administration of β-blockers (propranolol) is prescribed. Release form and packaging 1 ml of the drug is poured into glass ampoules. A marking text is applied to the ampoule with paint or a label is pasted. 5 or 10 ampoules, together with instructions for medical use in the state and Russian languages and a ceramic cutting disc, are placed in a cardboard box with partitions. 5 ampoules are placed in a blister pack made of polyvinyl chloride film and aluminum foil. One or two blister packs of 5 ampoules each, together with instructions for medical use in the state and Russian languages and a ceramic cutting disc, are placed in a cardboard box. It is allowed when packaging the drug in ampoules with a break ring or with a dot and a notch, do not insert a ceramic cutting disk. Storage conditions Store in a place protected from light, at a temperature from 8 0C to 15 0C. Keep out of the reach of children! Shelf life: 2 years Do not use the drug after the expiration date indicated on the package! Conditions for dispensing from pharmacies By prescription Manufacturer LLC “Pharmaceutical Company “Zdorovye””, Ukraine Owner of the registration certificate LLC “Pharmaceutical Company “Zdorovye””, Ukraine Address of the organization that accepts claims from consumers on the quality of products (products) on the territory of the Republic of Kazakhstan Address: LLC “Pharmaceutical” Health company. Ukraine, 61013, Kharkov, st. Shevchenko, 22. Phone Fax Email
Analgin
Rр.: Tab. A nalgini
0.5 N 10
DS 1 tablet 2-3 times a day
Rр.: Sol. Аnalgini 50% 1 ml
Dtd N 6 in ampullis.
S. 1 ml IM.
Gr.: non-narcotic analgesic, pyrazolone derivative.
Pok.: for pain of various origins (headache, neuralgia, radiculitis, myositis), feverish conditions, influenza, rheumatism, chorea.
MD, FS: blocks cyclooxygenase, reduces the formation of prostaglandins, thromboxane A2, providing an analgesic, antipyretic and mild anti-inflammatory effect.
Buy Adrenaline hydrochloride injection solution 0.1% 1ml No. 5 in pharmacies
Buy Adrenaline hydrochloride in pharmacies Adrenaline hydrochloride in the drug directory DOSAGE FORMS solution 0.1%
MANUFACTURERS Moscow endocrine plant (Russia) Ferein (Russia)
GROUP Drugs that stimulate alpha and beta adrenergic receptors
COMPOSITION Active substance: Epinephrine.
INTERNATIONAL NON-PROPENTED NAME Epinephrine
SYNONYMS Adrenaline, Synthetic adrenaline, Adrenaline hydrochloride-Vial
PHARMACOLOGICAL EFFECT Cardiostimulating, vasoconstrictor, hyperglycemic, hypertensive. Stimulates alpha and beta adrenergic receptors. When administered parenterally, it is very quickly destroyed by monoamine oxidase and catechol-O-methyltransferase in liver cells, kidneys, intestinal mucosa, and axons. The half-life is 1-2 minutes. Excretion of metabolites is carried out by the kidneys. It has a pronounced effect on the smooth muscles of internal organs, the cardiovascular and respiratory systems, and activates fat and carbohydrate metabolism. The first phase of action is primarily due to stimulation of beta-adrenergic receptors of various organs and is manifested by tachycardia, increased cardiac output, increased excitability and conductivity of the myocardium, arteriolo- and bronchodilation, as well as decreased uterine tone, inhibition of allergic reactions and mobilization of glycogen from the liver and fatty acids from fatty acids. depot Then alpha-adrenergic receptors are excited and the vessels of the abdominal organs, skin, mucous membranes, and, to a lesser extent, skeletal muscles narrow, blood pressure (mainly systolic), and general peripheral vascular resistance increase; the pressor effect can cause a short-term reflex slowing of the heart rate.
INDICATIONS FOR USE Anaphylactic shock, bronchospastic syndrome, hypoglycemia due to insulin overdose, open-angle glaucoma.
CONTRAINDICATIONS Arterial hypertension, widespread atherosclerosis, thyrotoxicosis, diabetes mellitus, angle-closure glaucoma, pregnancy. Adrenaline should not be used during anesthesia with fluorotane or cyclopropane (due to the development of arrhythmias).
SIDE EFFECTS Increased blood pressure, cardiac arrhythmias, ventricular fibrillation, anxiety, tremor of skeletal muscles, angina pectoris, tachycardia, headache, nausea.
INTERACTION Enhances the effect of aminophylline and drugs that increase thyroid function.
METHOD OF APPLICATION AND DOSAGE Therapeutic doses of adrenaline hydrochloride for parenteral administration are usually 0.3-0.5-0.75 ml of a 0.1% solution for adults. Depending on age, children are administered 0.1-0.5 ml of solution. Higher single doses: 0.1% solution of adrenaline hydrochloride and 0.18% solution of adrenaline hydrotartrate for adults under the skin: single 1 ml, daily 5 ml.
OVERDOSE No information available.
SPECIAL INSTRUCTIONS For the prevention of arrhythmias against the background of epinephrine, beta-blockers are indicated
STORAGE CONDITIONS List B. Store in a cool, dry place.
Anaprilin
Rp: Tab. Anaprilini
0.02 N 10
DS 2 tablets each. 3-4 times a day.
Rp: Sol. Anaprilini 0.25% – 1 ml
Dtd N 6 in ampullis.
S. IV slowly.
Gr.: -adrenolytic non-selective action
Pok.: for the treatment of coronary heart disease, heart rhythm disorders.
MD, FS: inhibition of the synaptic effect on the heart (decreased heart rate and decreased contractility), reduce cardiac output, decreased production and secretion of renin in the kidneys, prevents the release of norepinephrine into the synaptic cleft.
Adrenaline hydrotartrate solution 1%
Dosage forms:
0.1% solution of adrenaline hydrochloride for injection in ampoules of 1 ml and in bottles of 30 ml; adrenaline hydrotartrate injection solution 0.18% in ampoules of 1 ml.
Pharmacological group:
drugs acting primarily on peripheral adrenergic processes.
Pharmacological properties
The effect of adrenaline is associated with the influence on a and b adrenergic receptors and largely coincides with the effects of excitation of the sympathetic nerves: it constricts the vessels of the abdominal organs, skin, mucous membrane and, to a lesser extent, the vessels of skeletal muscles; adrenaline increases blood pressure, increases and increases heart rate; in connection with the increase in pressure, a reflex excitation of the center of the vagus nerves occurs, which has an inhibitory effect on the heart, as a result of which the heart rate can slow down. Adrenaline can cause heart rhythm disturbances (tachycardia, extrasystole), especially with coronary heart disease, as well as during anesthesia during operations.
Adrenaline relaxes the muscles of the bronchi and intestines, dilates the pupils, improves the functional activity of skeletal muscles, increases blood sugar, enhances tissue metabolism, and increases the myocardium's need for oxygen.
In terms of the action of adrenaline, hydrotartrate does not differ from adrenaline hydrochloride, but due to the difference in relative molecular weight, hydrotartrate is used in a higher concentration to obtain the same effect.
INDICATIONS FOR USE
Adrenaline hydrochloride and adrenaline hydrotartrate are used for anaphylactic shock, allergic laryngeal edema, and to relieve acute attacks of bronchial asthma; allergic reactions that develop when using drugs or other allergens, in patients with acute myocardial infarction with ventricular fibrillation, refractory to electrical defibrillation, and sudden cardiac arrest (asystole); with acute left ventricular failure; as a local vasoconstrictor.
CONTRAINDICATIONS
Adrenaline is contraindicated in hypertension, aneurysms, severe atherosclerosis, bleeding, and pregnancy. Adrenaline should not be used during anesthesia with fluorotane, cyclopropane, or chloroform (risk of developing arrhythmia).
SPECIAL INSTRUCTIONS
Use with caution in diabetes mellitus and hyperthyroidism.
METHOD OF APPLICATION AND DOSES
Adrenaline is administered subcutaneously, intramuscularly, intravenously (drip slowly) 0.2 - 0.3 - 0.5 - 1 ml of 0.1% hydrochloride solution or 0.18% hydrogen tartrate solution, intracardially in case of acute cardiac arrest - 1 ml and for ventricular fibrillation - 0.5 - 1 ml, and also applied to the mucous membranes as a local vasoconstrictor.
During an attack of bronchial asthma, solutions of adrenaline are administered 0.3 - 0.5 - 0.7 ml subcutaneously.
Therapeutic doses of solutions of adrenaline hydrochloride 0.1% and hydrogen tartrate 0.18% for parenteral administration are usually 0.3 - 0.5 - 0.75 ml for adults; Children, depending on age, are administered 0.1 - 0.5 ml. Higher doses for adults under the skin: single - 1 ml, daily - 5 ml.
SIDE EFFECT
Administration of adrenaline hydrochloride and hydrogen tartrate can cause increased blood pressure, tachycardia, arrhythmia, and pain in the heart area. For rhythm disturbances caused by adrenaline, beta-blockers (anaprilin, obzidan, etc.) are prescribed.
RELEASE FORM
Adrenaline hydrochloride is produced in the form of a 0.1% solution, adrenaline hydrotartrate - in the form of a 0.18% solution in ampoules, 1 ml of neutral glass and in hermetically sealed orange glass bottles (for topical use) of 30 ml.
STORAGE CONDITIONS
List B. In a cool (12-15°C), protected from light place. Keep out of the reach of children.
BEST BEFORE DATE
3 years. Do not use after the expiration date indicated on the package. Solutions that have turned brown or contain sediment are not suitable for use.
CONDITIONS OF VACATION FROM PHARMACIES
The drug is used in a hospital.
Adrenaline hydrochloride-Vial
During infusion, a device with a measuring device should be used to regulate the rate of infusion.
Infusions should be carried out into a large (preferably central) vein.
Intracardiac administration during asystole, if other methods are not available, because there is a risk of cardiac tamponade and pneumothorax.
During the treatment period, it is recommended to determine the concentration of K+ in the blood serum, measure blood pressure, diuresis, IOC, ECG, central venous pressure, pressure in the pulmonary artery and wedge pressure in the pulmonary capillaries.
Excessive doses during myocardial infarction may increase ischemia by increasing myocardial oxygen demand.
Increases glycemia, and therefore, diabetes mellitus requires higher doses of insulin and sulfonylurea derivatives.
When administered endotracheally, absorption and final plasma concentrations of the drug may be unpredictable.
The administration of epinephrine during shock does not replace transfusions of blood, plasma, blood substitute fluids and/or saline solutions.
Epinephrine is not advisable for long-term use (constriction of peripheral blood vessels, leading to the possible development of necrosis or gangrene).
There are no strictly controlled studies of the use of epinephrine in pregnant women. A statistically consistent relationship has been established between the appearance of deformities and inguinal hernia in children whose mothers used epinephrine during the first trimester or throughout pregnancy; it was also reported in one case that anoxia occurred in the fetus after intravenous administration of epinephrine to the mother. Epinephrine should not be used in pregnant women with blood pressure above 130/80 mmHg. Experiments on animals have shown that when administered in doses 25 times higher than the recommended dose for humans, it causes a teratogenic effect.
When used during breastfeeding, the risks and benefits should be assessed due to the high likelihood of side effects in the child.
Use to correct hypotension during labor is not recommended as it may delay the second stage of labor; when administered in large doses to weaken uterine contractions, it can cause prolonged uterine atony with bleeding.
Can be used in children with cardiac arrest, but caution should be exercised as the dosing regimen requires 2 different concentrations of epinephrine.
When stopping treatment, the dose should be reduced gradually, because Abrupt discontinuation of therapy may result in severe hypotension.
Easily destroyed by alkalis and oxidizing agents.
If the solution has turned pinkish or brown in color or contains sediment, it should not be administered. The unused portion should be destroyed.