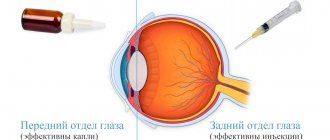
Medicines that reduce blood clotting include two pharmacological groups: antiplatelet agents and anticoagulants. They are very similar to each other in terms of the purpose for which they are prescribed - prevention of intravascular thrombus formation.
They differ from each other, in addition to the mechanism of action, the duration of use. Antiplatelet agents are prescribed, as a rule, for life; anticoagulants can be prescribed in short courses until the threat of thrombosis disappears, or for life, for example, after heart surgery.
In any case, the tactics of using drugs to reduce blood clotting are determined solely by the doctor.
Below we describe how these drugs work, what they are, and in what cases they are prescribed. The information presented is intended for informational purposes only and cannot be used as a guide for making a decision about the use of a particular medicinal product.
The importance of blood density readings for varicose veins
Thick blood with varicose veins is a fairly common occurrence. Standing for long periods of time, heavy physical activity, excess weight and pregnancy are the main reasons for the development of varicose veins. Blood cells slow down their circulation; more power is required to liquefy and pump them, which negatively affects the general condition of the blood vessels. After making an accurate diagnosis, the doctor prescribes certain medications that will improve microcirculation and normalize lymph outflow. Medicines also maintain normal hemostasis in the patient’s body, reduce blood clotting and destroy blood clots.
Viscous, thick blood cannot supply all tissues and organs with oxygen and other useful substances in a timely manner. This leads to numbness of the lower extremities, hypoxia and the active development of varicose veins. Platelets sticking to the walls of veins interfere with normal blood flow. To avoid blood clot rupture and death as a result of pulmonary embolism, it is necessary to take drugs to thin blood clots.
The effect of coagulants on the condition of blood vessels in varicose veins
The scientific name for blood thinners is anticoagulants. They do not get rid of the cause of the disease, but effectively relieve symptoms, have a strengthening effect on the walls of blood vessels, and block the progressive development of varicose veins. Any drug from this pharmacological group is taken strictly as prescribed by a phlebologist after a detailed ultrasound diagnosis.
Anticoagulants are indicated for the following diseases:
- Thrombosis of venous vessels;
- Arterial thrombosis;
- Pulmonary embolism;
- Other cardiovascular diseases associated with the formation of blood clots.
The active components of blood thinning drugs can be synthetic or semi-synthetic substances based on natural stimulants. The most effective are diosmin, heparin, troxerutin, rutin, escin and hesperidin. Each of them stabilizes the condition of blood vessels with varicose veins, reduces capillary fragility and prevents the formation of blood clots.
Contraindications for taking anticoagulants and antiplatelet agents
Like any medications, anticoagulants have a number of contraindications that must be carefully studied before starting to take the pills.
- Individual intolerance to the main components of the drug;
- Low blood clotting;
- The presence of open wounds or trophic ulcers;
- Phlebitis and hemorrhagic diabetes;
- 1st trimester of pregnancy;
- Hemophilia.
The role of platelets in primary hemostasis and atherothrombosis
The central pathological link of atherothrombosis is the so-called unstable plaque (Fig. 1),
Figure 1. Stages of atherosclerotic plaque formation [1]. a — unstable flat plaque without rupture; b — plaque rupture with non-occlusive thrombus and early organization; c - eroded flat plaque; d — eroded plaque with an occluding thrombus; d — hemorrhage inside the plaque; e - unstable plaque with a calcified focus; g - critically stenotic plaque with severe calcification. containing an atheromatous core. During the process of atherogenesis, macromolecules albumin, fibrinogen and lipoproteins penetrate into the plaque. These molecules form large aggregates in the extracellular matrix and are partially endocytosed by macrophages. Thus, the atheromatous core is avascular, almost acellular, devoid of supporting collagen, rich in extracellular lipids, mainly cholesterol and its esters, and has a soft “mush” consistency. An unstable plaque, unlike a stable plaque, has a thin covering, so it is susceptible to rupture. Plaque instability is thought to be due to an inflammatory response.
Violation of the integrity of an unstable plaque is facilitated by an increase in intravascular pressure and tone of the coronary arteries, tachycardia and damage to the vessels supplying it. The rupture usually occurs at the border of the plaque and the “healthy” part of the vessel. The contents of the plaque come into contact with the blood, which leads to activation of platelets and generation of a blood clot.
The formation of a platelet plaque at the site of vascular damage occurs in three stages:
1) the initial phase, including platelet adhesion;
2) activation and aggregation phase;
3) phase of stimulation of platelet receptors and stabilization of the clot.
The clinical manifestation of the formation of a “white” platelet-rich thrombus that partially or completely blocks the lumen of a coronary vessel is unstable angina (UA) and non-ST segment elevation myocardial infarction (NSTEMI). In myocardial infarction (MI) with ST segment elevation, a thrombus, predominantly “red” (rich in fibrin, dense), stably occludes the lumen of the vessel [2].
The main antiplatelet drugs act on different mechanisms of platelet activation (Fig. 2).
Figure 2. Mechanisms of platelet activation and action of antiplatelet drugs.
Antiplatelet drugs
The mechanisms of action of drugs that can influence the process of atherothrombosis are realized through their effect on enzymes or receptors that are necessary for the synthesis, activation and functional activity of important mediators. Common and studied oral antiplatelet drugs target key pathways of platelet activation.
Acetylsalicylic acid (ASA, aspirin) irreversibly inhibits cyclooxygenase type 1 by acetylating serine at position 529, thereby inhibiting the production of thromboxane A2, an activator of platelet aggregation, and prostaglandin I2. Prostaglandin I2 is a potential inhibitor of platelet aggregation and a potent vasodilator.
Clinical trials [3–5] have shown the effectiveness of ASA in the secondary prevention of MI, stroke and cardiovascular death.
6 large randomized trials evaluating the effectiveness of ASA for long-term therapy after acute MI demonstrated a reduction in mortality when taking ASA. A meta-analysis of these and many other studies by The Antithrombotic Trialist' Collaboration [3] demonstrated a 25% reduction in the incidence of cerebrovascular accidents, myocardial infarction, and cardiovascular death in the treatment group.
The Women's Health Study [6] included 39,876 female volunteers over 49 years of age who took 100 mg of ASA daily. Over a 10-year follow-up period, there was no significant effect of ASA on reducing the risk of MI or cardiovascular death, except in the subgroup of women over 65 years of age.
A systematic review by the Antithrombotic Trialist' Collaboration of 6 clinical trials of ASA in primary prevention included 95,000 patients. It was shown that in the ASA group, the risk of developing severe fatal complications decreased from 0.57 to 0.51% within a year due to a decrease in the incidence of non-fatal MI. The analysis also revealed a slight increase in the incidence of hemorrhagic strokes and a decrease in the incidence of ischemic strokes. There was an increase of 0.03% during the year in the incidence of gastrointestinal and extracranial bleeding [7].
Thus, ASA has now been proven effective in the secondary prevention of cardiovascular complications (CVC), but its use is not recommended for primary prevention due to the high risk of bleeding. In addition, at present, ASA is practically excluded from the European recommendations for atrial fibrillation and, according to domestic recommendations of 2011, can only be prescribed to women under 65 years of age in the absence of other risk factors for stroke and thromboembolism.
P2Y12 receptor antagonists include ticlopidine, clopidogrel, prasugrel and ticagrelor, as well as cangrelor and elinogrel. Ticlopidine, clopidogrel and prasugrel represent 3 generations of thienopyridines that selectively and irreversibly inhibit P2Y12 receptors. Newer drugs reversibly inhibit P2Y12 receptors and include an oral form (ticagrelor), an intravenous form (cangrelor), and both (elinogrel).
Clopidogrel is a member of the second generation of thienopyridines, which is metabolized by the CYP system in the liver. Clopidogrel was the first drug studied in a large cohort of patients with a history of ischemic stroke, primary myocardial infarction, and symptomatic peripheral arterial atherosclerosis in the CAPRIE trial (Clopidogrel versus Aspirin in Patients at Risk for Recurrent Ischemic Events). This study demonstrated the superiority of clopidogrel over aspirin in reducing the risk of recurrent ischemic events (p=0.043) [8]. In addition, the clinical superiority of clopidogrel in dual antiplatelet therapy (aspirin + clopidogrel) in patients with acute coronary syndrome (ACS) without ST-segment elevation was shown in the CURE study (20% reduction in the risk of coronary events in the clopidogrel group in NS; p<0.001). The COMMIT study [9] included 45,852 patients and showed a 9% reduction in the combined risk of death, recurrent myocardial infarction, and stroke in the clopidogrel group for myocardial infarction (p = 0.002). The CLARITY study [10] (clopidogrel as an adjunct to reperfusion therapy), which included 3491 patients, showed the benefit of clopidogrel in reducing the risk of developing cardiovascular events (p<0.001).
Antiplatelet agents are of particular importance during percutaneous coronary interventions (PCI), which are one of the most common methods of treating coronary heart disease [11, 12]. Performing PCI is accompanied by the risk of iatrogenic damage to the vascular wall [13], which leads to activation of the endothelium, loss of its thromboresistant properties and platelet hyperreactivity [14]. It is the specifics of interventional technologies that justify the clinical need (prevention of thrombotic complications) of more aggressive antithrombotic therapy, both in the early and late periods after the intervention [15].
The PCI-CURE study [16] demonstrated that in patients with non-ST-segment elevation ACS undergoing PCI, pre-procedural clopidogrel in addition to ASA followed by long-term use reduced the risk of CV events by 31% compared with placebo (p= 0.002). The potential effect of this combination was further confirmed in the CREDO study, which showed a relative reduction in the total risk of death, MI and stroke by 26.9% after 1 year of follow-up [17].
However, in patients with verified vascular atherosclerosis, the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Stabilized Ischemia) trial, which included 15,603 patients, showed that the combination of aspirin and clopidogrel was not significantly more effective than aspirin in reducing the incidence of MI, stroke, or death from cardiovascular events (p =0.22), but caused more bleeding. When analyzing the main subgroup of the CHARISMA study with documented MI, stroke, or clinically manifested peripheral arterial atherosclerosis, a benefit of dual-component antiplatelet therapy was revealed (reduced risk of MI, stroke, or cardiovascular death by 8.8% in the clopidogrel group; p = 0.01 ). A greater advantage was found in patients who had suffered an MI, in whom the reduction in the risk of developing recurrent CV events was 23% (p = 0.031). Although combination therapy with aspirin and clopidogrel had advantages in the secondary prevention of ischemic complications, CV events were re-reported in many patients, partly due to significant individual variation in platelet response to clopidogrel. It was suggested that the recommended doses of clopidogrel were ineffective, which led to a number of studies with increasing doses to 600 and 900 mg.
In vivo, it was shown that increasing the loading dose of clopidogrel to 600 mg leads to an increase in the concentration of its active metabolite and an increase in the antiplatelet effect. A further increase in the dose to 900 mg did not produce any additional effect [18]. According to the results of the CURRENT study published in 2010 [19], which included about 25 thousand patients, doubling the loading dose of clopidogrel improved the outcome only for patients receiving invasive treatment (reduction in the number of cardiovascular events by 13%). There was no significant difference in the incidence of the primary composite outcome (cardiovascular death, MI, or stroke) at 30 days between patients with ACS receiving double-dose or standard-dose clopidogrel. However, in patients undergoing PCI, double-dose clopidogrel was associated with a significant (42%) reduction in the incidence of stent thrombosis compared with patients using standard doses. The described positive effects were, however, offset by a relative increase of 25% in the incidence of hemorrhagic complications. Thus, a loading dose of clopidogrel 600 mg can only be indicated during PCI, and its administration is accompanied by an increased risk of bleeding.
One of the ways to combat resistance to clopidogrel was an attempt to introduce into practice the study of genes responsible for the absorption, metabolism and receptor sensitivity of platelets. According to recent recommendations, genetic testing is not indicated for routine use, but may be used in some cases. In accordance with the recommendations of the Clopidogrel Pharmacogenomics Consortium (2011), pharmacogenetic testing to determine alleles of the CYP2C19 gene can be carried out in the following situations:
— percutaneous interventions on the unprotected trunk of the left coronary artery;
— presence of bifurcation stenosis of the trunk of the left coronary artery;
- stenosis of the only patent coronary artery;
— history of thrombosis of the stent/stents;
— the patient has concomitant diseases that increase the risk of stent thrombosis: diabetes mellitus and chronic renal failure.
The third generation thienopyridine prasugrel, as well as direct reversible P2Y12 receptor antagonists - ticagrelor, cangrelor and elinogrel, are superior to clopidogrel in antiplatelet activity, are characterized by an earlier onset of effect and (with the exception of prasugrel) its rapid cessation after discontinuation, and the presumed absence of genetic resistance [20-22] . In recent years, a number of large studies have been conducted involving tens of thousands of patients comparing these drugs with clopidogrel. Prasugrel (TRITON TIMI 38 study) and ticagrelor (PLATO study) demonstrated superiority over clopidogrel in reducing the incidence of CV events and stent thrombosis during follow-up, but this effect was accompanied by an increase in the incidence of bleeding [23]. Ticagrelor demonstrated a more favorable benefit-risk ratio in this regard. Cangrelor, despite its beneficial pharmacological properties, was inferior to clopidogrel in two phase III studies (CHAMPION-PCI and CHAMPION-PLATFORM), and the studies were stopped early. Elinogrel is the only existing antiplatelet drug that can be administered either intravenously or orally. This makes it possible to quickly achieve pronounced suppression of platelet aggregation at the beginning of treatment (intravenous administration), followed by transition to oral administration after stabilization of the patient's condition. The drug is currently being studied in phase II clinical trials [24].
Platelet receptor glycoprotein blockers IIb/IIIa
Drugs of this class block the final stage of the platelet aggregation process - the binding of activated platelets to each other by fibrinogen, which attaches to platelet glycoprotein (GP) IIb/IIIa receptors [25]. With the advent of a large number of potent antiplatelet drugs acting at earlier stages of platelet aggregation, interest in GP IIb/IIIa receptor blockers has decreased somewhat in recent years.
Three representatives of this group are registered in the Russian Federation - abciximab, eptifibatide and the domestic drug monafram, which are administered intravenously, and due to the short half-life, therapeutic concentrations are achieved by continuous infusion.
According to the results of a pooled analysis of placebo-controlled studies (2002), GP IIb/IIIa receptor blockers demonstrated a relative reduction of 8% in the risk of NS and NSTEMI. The advisability of adding GP IIb/IIIa platelet receptor blockers to clopidogrel for UA/NSTEMI was less clear. According to the results of several large multicenter studies, which included a total of more than 30 thousand patients [26], the studied combination had an advantage over clopidogrel only in patients with UA/NSTEMI who underwent coronary revascularization (relative risk reduction by 10-20% , the most significant - in the presence of an increase in the level of cardiac-specific troponins).
In patients with UA/NSTEMI at risk (TIMI, GRACE or PURSUIT scales), three-component therapy is used: aspirin, clopidogrel, GP IIb/IIIa platelet receptor blocker. When choosing between a GP IIb/IIIa blocker and clopidogrel, the latter is preferred in case of a high risk of bleeding [27].
Dipyridamole is a pyrimidopyrimidine derivative with antithrombotic and vasodilating properties. It has been proven that the antithrombotic effects of dipyridamole are due to several mechanisms, including inhibition of cyclic guanosine monophosphate (cAMP), phosphodiesterase (PDA) type 5. However, this drug is not used in practical cardiology and is not included in the recommendations for ACS.
New antiplatelet drugs are currently being developed. Selective inhibition of major protease-activating receptor type 1 for thrombin, a major platelet activator, represents a promising new strategy for reducing ischemic complications without increasing the risk of bleeding. Two PAR-1 receptor antagonists are being tested in clinical trials: vorapaxar (SCH 530348) and atopaxar (E5555).
The findings led to a revision of the 2012 European guidelines on antiplatelet therapy. Thus, recommendations for the management of patients with ST-segment elevation MI indicate preference for prescribing a combination of aspirin with prasugrel or aspirin with ticagrelor over a combination of aspirin with clopidogrel. Clopidogrel should be used only in cases where patients, for one reason or another, cannot take the more powerful P2Y12 platelet receptor inhibitors - ticagrelor and prasugrel. In case of intolerance to aspirin, clopidogrel may be an alternative for long-term use.
However, the use of prasugrel has a number of significant limitations: it is not recommended for use in patients over 75 years of age, as well as with a body weight of less than 60 kg and a history of indications of a stroke or transient cerebrovascular accident. Currently, this drug is not registered in the Russian Federation.
As for ticagrelor, statistically significant differences in the incidence of adverse events identified in the PLATO (Study of Platelet Inhibition and Patients Outcomes) study - shortness of breath (13.8% versus 7.8%), episodes of asystole 3 s or more (5.8 % versus 3.6%) and increased levels of creatinine and uric acid require further, longer-term studies.
In addition, the cost of new antiplatelet drugs (ticagrelor and prasugrel) remains high, which is a significant obstacle to their widespread use and at the same time leaves open the possibility of using clopidogrel.
Thus, modern antiplatelet drugs developed in recent years are superior to their predecessors in effectiveness. At the same time, the main problem of intensifying antiplatelet therapy is the increased risk of hemorrhagic complications, as well as the presence of resistance in some patients. Future research in this area will be aimed at developing more effective antiplatelet drugs, with the least risk of bleeding and without the phenomenon of resistance.
Blood thinner for varicose veins
Blood thinning is one of the most important tasks in the complex treatment of varicose veins. At the same time, at each stage of the development of the disease, it is worth selecting certain drugs with a higher proportion of the active substance. Blood clots in damaged areas of the veins pose a dangerous threat to the normal nutrition and functioning of vital organs. Therefore, treatment of varicose veins is most effective in the first stages, when vascular damage has not acquired irreversible consequences.
Medicines to thin blood clots have 2 main directions:
- Anticoagulants - prevent the formation of fibrin clots by inhibiting the activity of proteins.
- Antiplatelet agents are drugs that prevent blood cells from sticking to each other.
Also, venotonics should be included in the complex of drug therapy to strengthen the walls of blood vessels, increase their tone and normalize blood flow in the damaged vein.
Anticoagulants
With varicose veins, the viscosity of blood cells is of great importance. The overall course of the disease and possible complications associated with the formation of blood clots will depend on this indicator. After a thorough history taking and ultrasound diagnostics, a phlebologist may prescribe medications that prevent the formation of blood clots - anticoagulants. They come in two types:
- Direct action - neutralizes risk factors for excessive coagulation, helps to dilute blood stagnation. Available in the form of injections or tablets (heparin).
- Indirect action - slowly reduce blood clotting factors, the medicine has a dose-dependent effect, so results can be observed after a course of taking the medicine. Representatives of this group of drugs are Aspirin and Warfarin.
Antiplatelet agents
Medicines in this group are capable of diluting venous fluid in varicose veins, preventing the “gluing” and “sticking” of red blood cells, and improving vascular patency. To prevent and treat the symptoms of thrombophlebitis, the following drugs are most often prescribed:
- Aspirin is a blood-thinning tablet that belongs to the group of non-steroidal anti-inflammatory drugs. Dispensed without a prescription, available in every pharmacy. Aspirin has many contraindications, so it should be taken in small doses under the strict supervision of the attending physician;
- Dipyridamole is a drug that can thin clots and inhibit platelet aggregation. Its pharmacological properties are similar to aspirin, but less dangerous due to contraindications.
- Warfarin is a medication for the treatment and prevention of thrombosis and varicose veins with a wide range of applications. It is a vitamin K antagonist, i.e. interferes with the active action of this vitamin.
Phosphodiesterase inhibitors
They influence another mechanism of blood clot formation. They have fewer contraindications and are considered safer when compared with the previous two pharmaceutical groups.
It makes sense to use them after emergencies, surgical interventions during the rehabilitation period, or as medications for the prevention of heart attack, stroke, and acute hemodynamic disorders associated with changes in blood properties.
Common names include Dipyridamole, Triflusal. Both are relatively old. They have several trade names that differ from the main ones, for example Curantil.
They often provoke allergic reactions, therefore they require careful prescription and monitoring of the patient’s condition.
Indications
{banner_banstat9}
It is impossible to say exactly when you need to take drugs of this type. The list of antiplatelet agents is wide, and the active ingredients also differ. It's worth taking a look at the instructions.
Theoretical speculations make no sense at all, because the question in any case falls on the shoulders of the doctor.
If you present the list on average, you will get the following picture:
- Transient ischemic attacks. Temporary episodes of circulatory disorders. Localization does not play a big role.
- Emergency conditions suffered in the recent past. Heart attack, stroke. In the first case, not everything is so obvious; many medications are not allowed in this situation. In the second one too.
We are talking only about the ischemic type of disorder. Not hemorrhagic, when there was hemorrhage.
- Stable high blood pressure. Hypertension.
- Cardiac surgery performed.
- Obliterating trophic disorders in the lower extremities. For example, atherosclerosis.
- Stroke prevention (read more about primary and secondary measures in this article).
- Coronary heart disease, except in some cases where the drug can cause harm.
The list is very approximate.
Side effects
There are relatively many of them. You should start from the name and group of the product. But the question is more transparent.
Particularly common violations among the possible ones:
- Prolonged bleeding that does not stop even after minimal damage: cuts, abrasions. This is almost impossible to avoid.
- Falling blood pressure levels.
- Dizziness, disorientation in space.
- Nausea, rarely progresses to vomiting.
- Allergic reactions. Perhaps the main side effect of the use of drugs to restore the rheological properties of blood.
The intensity varies. It is minimal when a rash forms on the skin before Quincke's edema or even anaphylactic shock. Fortunately, the last option occurs as an exception.
If negative phenomena develop, it makes sense to reconsider the course and regimen of treatment or completely abandon drugs of this type, which is also more of an unfortunate rare case than the rule.
Patients are advised to closely monitor their well-being. If adverse events develop, consult your doctor again.
Other drugs
These include those used for long-term treatment of abnormalities: Pentoxifylline (the most popular in clinical practice), Reopoliglucin (identical to the previous one, but safer and used in a wide range of cases).
Another type is complex medicines, which contain several components.
For example, Cardiomagnyl (Aspirin and magnesium, respectively), Aspigrel, Coplavix, Agrenox and others. The doctor decides whether it is worth prescribing such “explosive mixtures”.
In most cases, precise dosing is required, so it is better to give preference to two separate items.
It is safer, more effective and provides the doctor with the tools to fully control the process.
Moreover, the price of such “hybrids” is quite high, which completely eliminates any objections on this score from manufacturers. The issue is decided at the discretion of the treating cardiologist.