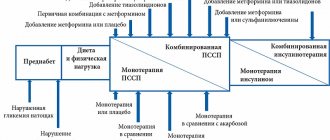
Metformin
Lactic acidosis
Lactic acidosis is a rare but serious (high mortality unless promptly treated) complication that may occur due to accumulation of metformin. Cases of lactic acidosis when taking metformin occurred mainly in patients with diabetes mellitus with severe renal failure.
Other associated risk factors should be taken into account, such as decompensated diabetes mellitus, ketosis, prolonged fasting, alcoholism, liver failure, any condition associated with severe hypoxia and simultaneous use of drugs that can cause the development of lactic acidosis (see section “Interaction with other drugs”) "). This may help reduce the incidence of lactic acidosis.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe asthenia. Lactic acidosis is characterized by acidotic shortness of breath, abdominal pain and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (less than 7.35), lactate content in the blood plasma over 5 mmol/l, increased anion gap and lactate/pyruvate ratio. If metabolic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
Metformin should be discontinued during surgery under general, spinal or epidural anesthesia. Metformin therapy can be continued no earlier than 48 hours after surgery or resumption of food intake, provided that renal function has been examined and found to be normal.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter, it is necessary to determine the clearance of creatinan:
- At least once a year in patients with normal renal function;
- Every 3-6 months in patients with creatinine clearance 45-59 ml/min.
- Every 3 months in patients with creatinine clearance 30-44 ml/min.
In case of creatinine clearance less than 30 ml/min, the use of the drug is contraindicated.
Particular caution should be exercised in case of possible impaired renal function in elderly patients, with dehydration (chronic or severe diarrhea, repeated bouts of vomiting), and with the simultaneous use of antihypertensive drugs, diuretics or non-steroidal anti-inflammatory drugs.
Heart failure
Patients with heart failure have a higher risk of developing hypoxia and renal failure. Patients with chronic heart failure should have their cardiac renal function monitored regularly while taking metformin.
Taking metformin in heart failure with unstable hemodynamic parameters is contraindicated.
Children and teenagers
The diagnosis of type 2 diabetes mellitus must be confirmed before starting treatment with metformin.
In clinical studies lasting 1 year, metformin was shown to have no effect on growth and puberty. However, due to the lack of long-term data, careful monitoring of the subsequent effects of metformin on these parameters in children, especially during puberty, is recommended. The most careful monitoring is necessary for children aged 10-12 years.
The use of iodine-containing radiocontrast agents
Intravascular administration of iodinated radiocontrast agents can lead to the development of renal failure and accumulation of metformin, which increases the risk of developing lactic acidosis. Metformin should be discontinued, depending on renal function, 48 hours before or during an X-ray examination using iodinated contrast agents, and not resumed until 48 hours after it, provided that during the examination, renal function was found to be normal.
Other precautions:
- Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day).
— It is recommended that routine laboratory tests be performed regularly to monitor diabetes mellitus.
— Metformin in monotherapy does not cause hypoglycemia, however, it is recommended to exercise caution when using it in combination with insulin or other hypoglycemic agents (for example, sulfonylurea derivatives, repaglinide, etc.).
The use of Metformin is recommended for the prevention of type 2 diabetes mellitus in persons with prediabetes and additional risk factors for the development of overt type 2 diabetes mellitus, such as:
— Age less than 60 years;
— Body mass index (BMI) ≥ 30kg/m:;
- History of gestational diabetes mellitus;
— Family history of diabetes mellitus in first-degree relatives;
- Increased concentration of HDL cholesterol;
- arterial hypertension.
— Metformin did not affect the fertility of male and female rats when administered at doses three times the maximum recommended daily dose for humans.
Metformin: new benefits in the light of evidence-based medicine
For more than 50 years, metformin has been used to treat type 2 diabetes mellitus. Its effectiveness and high safety profile have been proven by the results of the British Prospective Study of Diabetes Mellitus. Today, metformin is the first-line drug for this category of patients. It has been established that in addition to antihyperglycemic, metformin also has other effects. For example, it is able to inhibit the proliferation of tumor cells in in vivo
, and in
vitro
, and also slow down the aging process. However, further research is needed to confirm these effects.
Introduction
Biguanides have been used for the treatment of diabetes mellitus (DM) for more than 50 years. The main representatives of this group of drugs are metformin and phenformin. However, phenformin was withdrawn due to an increased risk of lactic acidosis with its use. Metformin increases lactate oxidation, but does not change its release from muscles or plasma concentrations [1, 2]. In this regard, metformin-induced lactic acidosis is extremely rare.
Since 2005, metformin has been a first-line drug for pharmacological treatment of type 2 diabetes (T2DM) according to the recommendations of the International Diabetes Federation (IDF), since 2006 - a first-line drug in combination with non-pharmacological treatment methods according to the recommendations of the American Diabetes Association (American Diabetes Association - ADA) and the European Association for the Study of Diabetes (EASD).
Currently, metformin is the most commonly prescribed oral hypoglycemic drug in many countries around the world [3]. That is why data on new properties of the drug are of particular interest [4].
Mechanism of action
The mechanism of the antihyperglycemic action of metformin has been well studied. It has been established that the drug does not affect the secretion of insulin by the beta cell; its effect is extrapancreatic. Metformin causes:
- decreased absorption of carbohydrates in the intestine;
- increased conversion of glucose into lactate in the gastrointestinal tract;
- increased binding of insulin to receptors;
- expression of the glucose transporter gene (GLUT) 1 (secretion);
- increased transport of glucose across the membrane in muscles;
- movement (translocation) of GLUT-1 and GLUT-4 from the plasma membrane to the surface membrane in the muscles;
- decreased gluconeogenesis;
- glycogenolysis;
- levels of triglycerides and low-density lipoproteins;
- increased levels of high-density lipoproteins.
The action of metformin is aimed at overcoming the resistance of peripheral tissues to insulin, primarily muscle and liver.
An important target of metformin is adenosine monophosphate-activated protein kinase (AMPK), a cellular energy sensor activated by metabolic stress. As a result of AMPK activation, hepatic glucose production is inhibited, insulin sensitivity and muscle glucose uptake are increased, and fatty acid oxidation occurs. This requires phosphorylation of threonine-172; the LKB1 gene is considered to be the responsible kinase. The downstream target of AMPK is the “mammalian target of rapamycin.” It is a kinase that regulates cell growth and is inhibited by AMPK, resulting in decreased protein synthesis [5].
AMPK can be activated by exercise, hormones, cytokines and drugs [5].
Activation of AMPK during metformin administration is time- and dose-dependent. It leads to inhibition of hepatic glucose production, increased muscle uptake, inhibition of acetyl-CoA carboxylase and decreased expression of fatty acid synthetase in rat hepatocytes [6] and possibly increased hepatic glucose utilization through insulin-independent pathways [7]. Activation of AMPK by metformin appears to be independent of changes in the adenosine monophosphate/adenosine triphosphate (AMP/ATP) ratio [7, 8] due to inhibition of the mitochondrial respiratory chain complex and an increase in reactive nitrogen species [8, 9]. At the same time, a study on mice showed that metformin can inhibit gluconeogenesis through an AMPK-independent pathway - through a change in the AMP/ATP ratio [10].
Another possible mechanism of antihyperglycemic action is the effect on incretin pathways. A. Maida et al. found that metformin acutely increased glucagon-like peptide 1 (GLP-1) levels in mice without concomitant glucose administration, but had no effect on other intestinal peptides such as peptide YY and glucose-dependent insulinotropic polypeptide. At the same time, metformin reduced the rate of gastric emptying and improved glucose tolerance, but this effect was not mediated by GLP-1 [11]. The effect of metformin on the activity of dipeptidyl peptidase 4 has not been established [11].
Metformin changes the metabolism of bile acids, influencing an increase in respiratory CO14 and fecal CO14 in patients with type 2 diabetes after administration of CO14-glycocholate [12]. Animal studies have shown that this effect is likely due to a decrease in the absorption of bile salts in the ileum [13] due to a decrease in active transport independent of Na-K-ATPase [14]. This could explain the gastrointestinal effects of metformin and its lipid-lowering properties. Therefore, it may be involved in glucose control, as bile acids activate the G protein-coupled receptor TGR5, which is able to stimulate the release of GLP-1 from L cells [15]. In the ME study, Patti et al. noted that patients who had undergone gastric bypass had higher levels of bile acids compared to overweight or obese patients, and this level was positively correlated with postprandial peak GLP-1 and negatively with glucose levels. Therefore, bile acids can stimulate GLP-1 [16].
The antihyperglycemic effect of metformin can also be explained by its effect on changes in islet amyloid deposits. Amyloid deposition is known to result in the reduction in beta cell mass that is common in type 2 diabetes. In a study of transgenic mice (expressing islet amyloid peptide), metformin reduced the prevalence and severity of islet amyloidosis, although it did not affect amyloid formation. It has been established that this effect may be associated with changes in body fat mass and insulin secretion [17].
British Prospective Study of Diabetes Mellitus
The most comprehensive evaluation of metformin treatment compared with other treatments comes from the United Kingdom Prospective Diabetes Study (UKPDS). The study included patients with newly diagnosed type 2 diabetes in the period 1977–1991. The number of participants is 5102. Of these, 59% are men and 41% are women. The body mass index of patients was 28 kg/m2, the level of fasting glycemia (median) was 11.5 mmol/l, glycated hemoglobin (median) was 9.1%. High blood pressure (BP) was observed in 39% of patients.
The main idea of UKPDS is to study the influence of hyperglycemia, elevated blood pressure and the main methods of their correction on the development of complications of diabetes. The study consisted of two parts: studying glycemic control and blood pressure control. The study of glycemic control had two objectives: the first was to assess whether improving blood glucose levels in type 2 diabetes could prevent the development of clinical complications of the disease, the second was to assess whether therapy with sulfonylureas (generation I or II), insulin or metformin.
After three years, metformin resulted in similar reductions in fasting plasma glucose and glycated hemoglobin as sulfonylureas or insulin. In addition, metformin reduced fasting plasma insulin levels and did not increase body weight [18]. Metformin therapy was accompanied by a lower frequency of hypoglycemic episodes compared to therapy with sulfonylureas and insulin, but was higher compared to diet therapy [19].
A ten-year analysis showed that the metformin group achieved glycemic control comparable to other groups without significant differences in body weight, but with lower fasting plasma insulin levels and a lower frequency of hypoglycemic episodes (compared to the sulfonylurea and insulin groups) [20].
When assessing the effect of treatment on the development of diabetes complications, the metformin group had a lower risk of any disease-related endpoint (32%): diabetes-related deaths (42%), total mortality (36%), myocardial infarction (39%) and all macrovascular diseases (30%) – compared to the diet therapy group. In the metformin group, compared with the traditional therapy group, a lower risk of progression of retinopathy was noted. There were no significant differences in nephropathy. When compared with the sulfonylurea and insulin groups, only the risks of diabetes-related endpoints and all-cause death were significantly lower in the metformin group [20].
The benefits of metformin were assessed only in overweight patients. An observational retrospective study that analyzed a database of patients treated with metformin or sulfonylureas for at least six months suggested that metformin may be as effective as sulfonylureas in obese and nonobese patients [21].
In summary, the UKPDS demonstrated that metformin is as effective as sulfonylureas and insulin for glycemic control, with a lower risk of hypoglycaemia.
Metformin and cancer
In recent years, the ability of metformin to reduce the risk of malignant neoplasms or death from them in patients with type 2 diabetes has been actively studied.
According to epidemiological studies conducted over the past decades, people with diabetes (primarily type 2 diabetes) are more predisposed to developing cancer. More often they are diagnosed with liver, kidney, pancreatic and breast cancer, colorectal cancer and uterine cancer. It should be noted that the relative risk varies depending on the location of the tumor – from 1.4 to 4.5 [22–24]. This may be explained by the hyperinsulinemia and insulin resistance characteristic of type 2 diabetes. Insulin is known to have mitogenic effects.
JM Evans et al. (2005) first drew attention to the fact that taking metformin can reduce the risk of malignant neoplasms [25]. Thus, an analysis of a database of 11,876 residents of the Tayside area (Scotland) who developed type 2 diabetes from 1993 to 2001 showed that 923 people were hospitalized for cancer at least one year after diagnosis. The average age of patients was 73 years, duration of diabetes was 8.4 years. Subsequent analysis, taking into account some “interfering” factors (smoking, body mass index, blood pressure, etc.), showed that in patients receiving metformin, the relative risk of malignant neoplasms was reduced by 23% compared with patients who did not take this drug. The authors did not provide data on the location of tumors.
In another epidemiological study, S. L. Bowker et al. (2006) showed that the incidence of cancer-related death was significantly lower in the metformin group compared to the sulfonylurea group [26].
Finally, in the work of GW Landman et al. (2010) noted that in 1353 patients with diabetes, observed for an average of 9.5 years, mortality from malignant neoplasms, the nature of which was not specified, among those taking metformin was significantly lower than among those who did not use this drug. The higher the daily dose of the drug, the higher the indicated effect [27].
Epidemiological data have prompted the study of the role of metformin in various types of cancer.
A retrospective cohort study conducted in the United States included 191,233 patients with diabetes. Average age: 56 years, 49% women. During an average of 3.9 years of follow-up, 813 cases of cancer were identified. Among the patients, a group was identified who received metformin monotherapy. In this group, the effect of taking the drug on the risk of developing bladder cancer was not recorded, but there was a tendency to increase the risk of developing pancreatic cancer and reduce the risk of developing colorectal cancer and liver cancer [28]. In two other, relatively small in terms of the number of patients, studies conducted in the USA and Italy, while taking metformin, on the contrary, a decrease in the risk of developing pancreatic cancer was observed [29] and a decrease in the risk of developing hepatoblastoma was confirmed - mostly due to men [30 ]. In a study by JL Wright et al. Metformin use was associated with a reduced risk of prostate cancer in Caucasian patients [31]. Another important but not fully understood fact is the lack of effect of metformin on the risk of developing breast cancer [28, 32], which could be expected based on some preclinical trials and which may be explained by various reasons [33, 34].
However, in the work of G. Libby et al. (observation period – 10 years), the use of metformin in patients with diabetes (over 4000 people) led to a 37% reduction in the risk of developing tumors in general and tumors of the lungs, colon and breast in particular [35].
In vitro studies
and animal studies confirm that metformin can prevent cancer.
Metformin and aging
A research group at the Catholic University of Leuven (Belgium) demonstrated that metformin can stop the aging process and prolong life. Scientists conducted a series of experiments with roundworms (Caenorhabditis elegans). This species serves as an ideal model for studying aging processes, since the life expectancy of its representatives is only three weeks. The work revealed that metformin increased the amount of toxic compounds released from cells. Perhaps this is what increased the lifespan of the worms. “As worms age, they become smaller, shriveled and begin to move less. But the worms that we gave metformin showed very limited reduction in size and did not shrink. They not only aged more slowly, but also remained healthy longer,” said study author W. de Haes. Scientists explain this effect of metformin by the fact that body cells receive energy from mitochondria - microscopic “power plants” that generate a very weak electric current. This process is accompanied by the formation of highly reactive oxygen species (radicals). They can damage proteins and DNA, interfering with the normal functioning of cells. However, in small concentrations these molecules can be useful. Metformin causes a slight increase in their number.
The US National Institute of Aging has found that metformin increases the life expectancy of laboratory mice. The average lifespan of animals is 150 days. While taking metformin, it increased to an average of 160 days. At the same time, animals treated with metformin had significantly better physical fitness, and the risk of developing age-related cataracts was also significantly reduced.
However, the biomolecular mechanism of the described action of the drug has not been established. There is only a similarity with the effect of a low-calorie diet. As a result of inhibition of electron transport of the mitochondrial respiratory chain, the concentration of intracellular ATP decreases and anaerobic glycolysis is triggered. This helps to reduce metformin fat oxidation and the synthesis of free fatty acids.
In order to achieve this effect, scientists had to significantly increase the dose of the drug (in proportion to the human dose), which can have a toxic effect on the body.
Director of the US National Institute of Aging R. Hodes commented on the results: “There is increased interest in drugs that can be repurposed for other purposes. It's a tremendous advance to discover that a long-used drug can be used for something else, such as healthy aging."
Conclusion
Today, metformin is the main drug for the treatment of type 2 diabetes. Although it has been used in clinical practice for more than 50 years, interest in the drug has not waned. Many studies of the last decade, devoted to various aspects of the use of metformin, demonstrate the uniqueness of the drug, reveal its potential and new possibilities for use.
Introduction
Diabetes mellitus (DM) is the most acute medical and social problem of our time, affecting many people, since the increase in incidence has led to talk about a global epidemic of diabetes.
According to the International Diabetes Federation (IDF), as of 2022, there will be 425 million people with diabetes in the world [1]. Type 2 diabetes (T2DM) accounts for 90% of cases. If treatment is insufficiently effective, T2DM can significantly limit the lives of patients, leading to early disability and premature mortality due to the development of vascular complications, which determine the severity of the disease and the damage caused to society. The consequences of diabetes cause enormous economic costs for healthcare systems, since about 80% of the costs of treating diabetes are associated with the treatment of vascular complications. Thus, the addition of diabetic complications on average increases the cost of treatment by 3–10 times [2]. Among the pathogenetic mechanisms of hyperglycemia, insulin resistance (IR) plays a key role, so it is not surprising that the successes achieved in the treatment of diabetes are largely associated with the use of drugs that improve insulin sensitivity [3]. The significant role of IR in the premature development of cardiovascular diseases (CVD) associated with atherosclerosis, as well as in increasing the risk of acute macrovascular complications, regardless of other significant risk factors, including hyperglycemia, dyslipidemia, and smoking has been established [4, 5].
Choosing IR, a key pathophysiological link in T2DM, as a strategic target for treatment can improve insulin sensitivity and have a positive effect on associated metabolic disorders. Pharmacological correction of IR increases the effectiveness of glucose-lowering therapy aimed at other pathophysiological links in the development of T2DM [6–8]. Biguanides have a pronounced inhibitory effect on IR. The history of their use began in the Middle Ages with the use of guanidine, which was obtained from the plant Galega officinalis (galega officinalis, French lily). However, severe toxicity prevented the clinical use of this substance [9].
Currently, metformin is the only biguanide recommended for pharmacotherapy of patients with T2DM. Due to its high clinical effectiveness, unique therapeutic properties, good safety profile, proven in clinical studies and confirmed by many years of experience in use, in modern clinical recommendations metformin has secured a leading position in the treatment of T2DM (provided there are no contraindications to it) and is used at all stages of the disease [ 8, 10, 11]. The clinical effects of metformin were first presented after the completion of the multi-year UKPDS (United Kingdom Prospective Diabetes Study) study in 1998 [12]: a reduction in the risk of vascular complications by 32%, myocardial infarction by 39%, diabetes mortality by 42% and overall mortality by 36%. Since 2005, metformin has been considered a first-line drug for the pharmacological treatment of T2DM in combination with non-pharmacological methods, according to the IDF recommendations; since 2006, according to the recommendations of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (European Association for the Study of Diabetes). Study of Diabetes – EASD) [13].
Mechanism of action and hypoglycemic (antihyperglycemic) effect of metformin
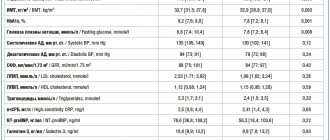
The antihyperglycemic effects of metformin are the result of the drug's effect on tissue sensitivity to insulin. Although the predominant effect of metformin is on hepatic glucose production, it is the combination of effects at the level of liver, muscle and adipose tissue that generally provides a favorable pharmacological profile [6, 7, 9]. It is known that the consequences of excess endogenous glucose production by the liver are especially unfavorable due to the stimulation of atherogenesis and the development of resistance to the action of glucose-lowering drugs during the day. In particular, with an increase in fasting blood glucose (FBG) >6.1 mmol/L, the risk of developing cardiovascular events over the next 12.4 years increases by 1.33 times [14]. The use of metformin leads to a 25–30% decrease in the level of GKN and postprandial glycemia (Table 1) [8, 13].
Several mechanisms are responsible for the antihyperglycemic effect of metformin, with its main effect occurring at the level of hepatocyte mitochondria [9, 13]. The molecular target of metformin is adenosine monophosphate-activated protein kinase (AMPK), known as a key enzyme in cellular metabolism and energy balance (see figure). Metformin-mediated activation of AMPK leads to the restoration of energy homeostasis by increasing glucose uptake in skeletal muscle and suppressing gluconeogenesis in the liver [15, 16]. Under the influence of metformin, the liver kinase B1 (LKB1)/AMPK signaling pathway is activated; in which LKB1 is an upstream activator of AMPK, responsible for phosphorylation of Thr 172 of the catalytic α-subunit of this kinase. Activation of AMPK is secondary to the effects of metformin on the mitochondrial respiratory chain. The following is considered the most likely mechanism explaining the activation of AMPK. AMPK is activated by phosphorylation of LKB1 kinase in response to an increase in the intracellular AMP/ATP ratio through inhibition of the mitochondrial respiratory chain and an increase in reactive nitrogen species under the influence of metformin. Activation of AMPK suppresses the expression of genes responsible for gluconeogenesis enzymes [15, 17].
The effects of insulin on the transcription, translation and synthesis of phosphatidylinositol 3-kinase, which is responsible for the translocation of glucose transporters to the plasma membrane, are enhanced, which leads to an increase in glucose uptake by liver, muscle and fat cells [6, 8]. In parallel with this, the oxidation of fatty acids occurs.
In muscle and adipose tissue, metformin increases the binding of insulin to receptors, and an increase in their number and affinity is also observed. In addition, the post-receptor mechanisms of insulin action, in particular tyrosine kinase and phosphotyrosine phosphatase, are activated [7, 10].
The study of the cellular and molecular mechanisms underlying the action of metformin showed that inhibition of the expression of gluconeogenesis genes is not the only mechanism of the hepatic effects of metformin (AMPK-independent mechanisms) [17]. Another concept is based on the close relationship between the energy profile of hepatocytes and hepatic glucose production, an energy-consuming process. Against the background of a decrease in ATP levels caused by metformin, hepatocytes have to correspondingly reduce glucose production [18].
Another mechanism of the antihyperglycemic action of the drug deserves discussion. Metformin modulates the activity of the incretin axis and increases glucagon-like peptide-1 (GLP-1) levels [17, 19, 20]. There are several hypotheses regarding how metformin promotes GLP-1 secretion. In particular, metformin stimulates GLP-1 secretion through activation of Wnt signaling pathways in intestinal L cells in db/db mice. In addition, it stimulates the expression of genes encoding GLP-1 receptors and glucose-dependent insulinotropic polypeptide (GIP) in the islets of Langerhans, and increases the expression of precursor protein genes - preglucagon, proglucagon [19]. Data regarding the effect of metformin on dipeptidyl peptidase-4 (DPP-4) activity are conflicting. In vivo studies, as opposed to in vitro studies, have shown that metformin use is associated with a decrease in DPP-4 levels (possibly through an effect on hepatic DPP-4 production).
There is an opinion about the indirect effects of metformin on the secretion of GLP-1, which are mediated by changes in the enterohepatic circulation of bile acids. Metformin inhibits nuclear farnesoid X receptors (FXR) by an AMPK-dependent mechanism. Acting as an AMPK activator, metformin suppresses the transcriptional activity of FXR. As a result, intestinal absorption of bile acids decreases and their pool in the ileum increases. Bile acids are natural ligands of the membrane bile acid receptor or TGR5 of intestinal enterochromophin cells. In turn, stimulation of TGR5 induces the secretion of GLP-1 by L cells. Activation of TGR5 increases energy expenditure and oxygen consumption, thereby improving insulin sensitivity [21].
Metformin and β-cell function
T2DM is considered a chronic progressive disease, therefore it is extremely important to maintain the functional activity of the β-cell apparatus of the pancreas [22]. It is fundamentally important that, while increasing hepatic and peripheral sensitivity to insulin, metformin does not affect insulin secretion [6, 10, 17]. At the same time, by increasing tissue sensitivity to the action of insulin, reducing gluco- and lipotoxicity, the drug indirectly improves insulin secretion and helps maintain the functional activity of β-cells. Thanks to all of these effects of metformin, a decrease in glucose levels occurs without the risk of hypoglycemic conditions, which is considered an undoubted advantage of the drug [23].
In this regard, the results of a retrospective study by SA Berkowitz et al. are interesting, in which glucose-lowering therapy was analyzed at the stage of its initiation and intensification, assessing data from more than 15.5 thousand patients [24]. According to the design, patients newly prescribed oral antihyperglycemic drugs (OHDs) between 2009 and 2013 were classified according to the treatment received into several groups: metformin only or sulfonylureas (SLUs), or thiazolidinediones, or DPP inhibitors only. 4.
The purpose of this study was to determine the timing of intensification of therapy (by adding another antidiabetic agent, including insulin) in patients with T2DM who were prescribed PSP for the first time. As it turned out, despite the existing treatment algorithms for T2DM, only 57.8% of patients began treatment for the disease with metformin. Using Cox regression analysis in a large patient population, the authors showed that starting therapy with metformin was associated with a lower need for intensification of therapy in the future compared with other DSPs (Table 2). In particular, the proportion of patients who required the prescription of a second antihyperglycemic agent and who received metformin at the start of therapy was 24.5%, DPP-4 inhibitors - 36.2%, PSM - 37.1%, thiazolidinediones - 39.6%.
By reducing the concentration and oxidation of free fatty acids (FFA) by 10–17 and 10–30%, respectively, and activating their reesterification, metformin not only improves tissue sensitivity to insulin, but also prevents the progression of impaired insulin secretion in patients with T2DM [7, 10, 13 ]. The consequences of excess production of FFAs boil down to disruption of their compensatory oxidation. As a result, it leads to the activation of non-oxidative metabolic pathways with the formation of metabolites that have a lipotoxic effect on tissues (liver, heart, muscle tissue, pancreas). In particular, excess FFA suppresses the activity of pyruvate dehydrogenase and disrupts the transport and phosphorylation of glucose in skeletal muscles. Increased concentrations of FFAs at the liver level stimulate gluconeogenesis. In turn, normalization of FFA concentrations during the use of metformin leads to the elimination of the effects of lipotoxicity at all levels, including the liver, adipose and muscle tissue, and the islets of Langerhans. A decrease in the supply of FFA to the liver, the synthesis of triglycerides, and an increase in insulin sensitivity are accompanied by a decrease in fat deposition in this organ [17, 18].
As is known, glucotoxicity is a key factor in the progression of β-cell dysfunction [22]. By influencing the absorption of carbohydrates in the gastrointestinal tract, slowing its rate, and also reducing appetite, metformin helps reduce postprandial glycemia, which also helps eliminate glucose toxicity. In addition, metformin increases the utilization of glucose in the intestine, enhancing anaerobic glycolysis in it both in a state of saturation and on an empty stomach. As a result, during metformin therapy, postprandial glycemia decreases by an average of 20–45% [17, 23].
Metformin and body weight
An important goal in the treatment of T2DM is to reduce and maintain normal body weight. According to clinical studies, metformin therapy prevents weight gain or promotes weight loss, incl. and in combination with insulin. Depending on the duration of drug use, the decrease in body weight in patients with T2DM ranges from 0.5 to 6.0 kg [9, 10, 13]. This remains a significant advantage of the drug, since the use of PSM usually causes weight gain. An interesting fact: metformin therapy is accompanied by a decrease in visceral fat deposition, which is an independent factor of CVD [25].
The potential effect of metformin on body weight is due to several mechanisms, including: decrease in insulin concentration, increase in GLP-1 secretion. According to the results of experimental studies on animal models, metformin has an anorexigenic effect, apparently mediated by the central action of the drug at the level of hypothalamic neurons [26]. Improving insulin sensitivity with metformin eliminates the cause of hyperinsulinemia, which also helps reduce appetite and reduce energy consumption (on average 104.5 kcal/day) [27].
The use of metformin is accompanied by suppression of the production of the orexigenic peptide ghrelin and an increase in the concentration of GLP-1, which has an anorexigenic effect, which partly explains some metabolic effects [17, 20, 25]. In addition, by modulating the expression of orexigenic neuropeptide Y, the drug promotes weight loss [28].
Non-glycemic effects of metformin
Currently, considerable attention is paid to the non-glycemic effects of glucose-lowering therapy, since it is important not only to achieve glycemic control, but also to influence other modifiable risk factors for the development of cardiovascular pathology to improve life prognosis.
When assessing the effectiveness of metformin, they often turn to glycemic control, but other effects (hypolipidemic, vasoprotective, etc.) of the drug also acquire considerable importance [6, 7, 10]. Approximately half of patients with T2DM have atherogenic dyslipidemia, which adversely affects cardiovascular prognosis. The effects of metformin on plasma lipid metabolism are due to its hypolipidemic and antiatherogenic effects. As already mentioned, metformin activates AMPK, resulting in the suppression of the expression of lipid metabolism genes, such as FFA synthetase (G001196), acetyl-CoA carboxylase (G000684), S14, sterol regulatory element binding protein 1 (SREBP-1c) [ 29]. In addition, metformin affects the biosynthesis of monounsaturated fatty acids from saturated fatty acids through AMPK-mediated phosphorylation of thyroid hormone receptor 4 (TR4) [17].
In addition to the effect on FFA metabolism, metformin treatment is associated with positive changes in the lipid spectrum; in particular, a 10–20% decrease in the concentration of triglycerides, a 10% decrease in low-density lipoproteins, and a 10–20% increase in the concentration of high-density lipoproteins [13, 17, 23]. Such effects serve as an additional advantage of metformin from the perspective of the main goal of T2DM therapy - reducing cardiovascular morbidity and mortality.
The information would be incomplete if we did not provide the results of experimental work indicating the effect of metformin on the metabolism of lipids in the vascular wall. In particular, the drug reduces the accumulation of cholesterol esters in the aorta, increases the content of phospholipids and reduces the content of sphingomyelin. Along with this, metformin reduces the proliferation of vascular smooth muscle cells (SMCs). By reducing the deposition of lipids in the vascular wall, reducing the proliferation of SMCs, disrupting adhesion, transformation of monocytes and the ability to take up lipids, metformin actively affects the early stages of the development of atherosclerosis [30]. It should be noted that, by suppressing the increased adhesion of monocytes to the vascular endothelium, lipoidosis, metformin affects the triggers for the development of atherosclerotic lesions and reduces the expression of receptors involved in the processes of intracellular lipid accumulation [31]. In addition, metformin suppresses the processes of differentiation of monocytes into macrophages, which actively secrete proatherogenic factors. In vitro, metformin has been shown to have an inhibitory effect on leukocyte-endothelial interaction, as well as the expression on the endothelial surface of such adhesion molecules as intracellular adhesion molecule 1, vascular cell adhesion molecule 1 and E-selectin [10, 31].
Additional cardioprotective effects of metformin are also due to its effect on the hemostatic system, blood rheology (antithrombic effect), endothelial function and vascular reactivity (Table 3).
In particular, metformin reduces the level of platelet activation markers (platelet factor 4 and β-thromboglobulin), the concentration of plasminogen activator inhibitor-1, the production of adhesion molecules and fibrinogen, and inhibits the activity of coagulation factors XIII and VII [8, 13, 30].
The vasoprotective properties of metformin are multifaceted: normalization of the contraction/relaxation cycle of arterioles, reduction of vascular wall permeability and inhibition of neoangiogenesis processes, improvement of endothelium-dependent vasodilation and glucose transport in endothelial cells, vascular SMCs, as well as in the myocardium [23, 32].
Metformin has a direct anti-inflammatory effect on the vascular wall, which is mainly due to the suppression of signaling pathways for the formation of NF-kB, the activation of which leads to the initiation of transcription of genes for a number of cytokines and adhesion molecules [17].
Taken together, the glycemic effects and additional properties (anti-sclerotic, vasoprotective, anti-inflammatory, antioxidant) of metformin are important for reducing the risk of CVD and increasing the life expectancy of patients with T2DM. Currently, T2DM is considered to be equivalent to the presence of clinically significant CVD in a patient, which places serious demands on the long-term cardiovascular safety of glucose-lowering drugs [5]. A good illustration of the cardioprotective properties of metformin are the results of the UKPDS study regarding overall and cardiovascular outcomes. For the first time, the beneficial effect of metformin on the incidence of CVD in patients with diabetes and obesity was established in the UKPDS study [12]. Subsequently, other studies showed a decrease in cardiovascular and overall mortality among patients with type 2 diabetes who received metformin alone or in combination with other glucose-lowering drugs [33–35]. In particular, the result of a Canadian retrospective analysis of a patient database (Saskatchewan Health databases, n=12,272) showed a significant reduction in overall and cardiovascular mortality by 40 and 36%, respectively [33]. In the PRESTO (Prevention of Restenosis with Tranilast and its Outcomes) study, metformin use was associated with an improved prognosis—a reduced risk of all clinically significant events (28%), myocardial infarction (69%), and all-cause mortality (61%) [34] .
Recently, the results of a retrospective analysis of the cardiovascular safety of 12,020 thousand patients with T2DM (the UK Clinical Practice Research Datalink) who were prescribed insulin therapy since 2000 were published [35]. The primary endpoint of the study was mortality from any cause, with secondary endpoints including myocardial infarction and stroke. As a result, the use of insulin in combination with metformin is associated with a 40% reduction in the risk of death from all causes compared with insulin monotherapy (hazard ratio - RR = 0.60, 95% confidence interval [CI] - 0.52-0.68 ). Among patients receiving insulin in combination with metformin, compared with patients on insulin monotherapy (n=6484), there was a 25% reduction in the risk of myocardial infarction and stroke (RR=0.75, 95% CI – 0.52–0.68 ).
More and more information about new targets of metformin is appearing in scientific publications. It has become apparent that the metabolic effects of metformin are partly due to changes in the intestinal microbiota [17, 20]. In particular, the use of metformin is accompanied by an increase in the number of bacterial species of the genus Escherichia, Akkermansia muciniphila, Subdoligranulum, incl. bacteria that produce short-chain fatty acids (butyrate), which stimulate the synthesis of incretins. Microbial analysis showed a significant decrease in the potential of the microbiota to produce butyrate in patients with T2DM [33, 37].
Other uses of metformin
The results of numerous experimental and clinical studies have made it possible to reveal new aspects of the action of metformin, to more fully assess its practical significance, and also to expand the indications for its use [15, 17, 18, 20].
In recent years, much attention has been paid to the anticarcinogenic effect of metformin, which most likely occurs through the activation of AMPK. This leads to inhibition of the activity of M-TOR (mammalian target of rapamycin), a central regulator of protein synthesis and cell growth, with subsequent restoration of insulin sensitivity and reduction of hyperinsulinemia [17, 39]. The insulin-lowering effect of metformin may play an important role in antitumor activity because Insulin has mitogenic and proliferative properties, and tumor cells often express high levels of the insulin receptor [38–40]. Metformin is also effective in the treatment of polycystic ovary syndrome [41].
Practical aspects of the use of metformin
Due to the absence of long hydrophobic side chains, both the ability of the drug to bind to the cell membrane and active accumulation inside the cell are limited, which determines the low likelihood of lactic acidosis and the high safety of metformin therapy [6, 9, 10]. Treatment with the drug is initiated with 500–850 mg taken with dinner or at night. To avoid the side effects of metformin (dyspeptic symptoms, diarrhea, abdominal discomfort), a gradual titration of the drug dose is necessary, increasing by 500–850 mg every 1–2 weeks, and in some cases a temporary reduction to the previous dose. Side effects usually disappear when the dose of the drug is reduced. The maximum recommended dose for patients with metabolic syndrome is 2500–3000 mg/day in 2–3 divided doses.
Metformin can be continued until the glomerular filtration rate is >45 ml/min/1.73 m² in the absence of other contraindications:
- diseases accompanied by tissue hypoxia (heart or pulmonary failure, myocardial infarction, anemia, etc.);
- renal failure or impaired renal function (creatinine clearance <45 ml/min/1.73 m²);
- liver failure, alcoholism;
- pregnancy, lactation;
- acute conditions that may impair renal function (dehydration, acute infection, shock, intravascular administration of radiocontrast agents);
- diabetic ketoacidosis.
You should refrain from prescribing the drug during pregnancy and lactation.
The results of clinical studies indicate that metformin reduces the level of glycated hemoglobin (HbA1c) by 0.6–2.4% [6–9]. This variability in the degree of glycemic reduction is associated with the initial blood glucose concentration before initiation of treatment with this drug. The failure to achieve the target HbA1c level during metformin monotherapy indicates a significant impairment of the secretory activity of β-cells. In these cases, it is necessary to use a combination of metformin with a glucose-lowering drug of a different mechanism of action. The mechanism of action of metformin makes it possible to successfully combine metformin with all hypoglycemic drugs (sulfonylureas, meglitinides, thiazolidinediones, GLP1 agonists, DPP4 inhibitors), which increases the overall therapeutic efficacy and improves glycemic control [8, 18, 23]. Separately, it should be noted that the combination of metformin and insulin is rational; with this option, tissue sensitivity to exogenous insulin is significantly improved. As a result, the daily dose of insulin is reduced by an average of 17–30%, the risk of hypoglycemia is reduced, and the risk of weight gain does not increase [13].
Conclusion
It is obvious that T2DM represents a serious health problem for the population of the Russian Federation. Solving this problem requires increasing the effectiveness of glucose-lowering pharmacotherapy, in which the use of drugs that affect IR is of great importance. Metformin is a highly effective and safe hypoglycemic drug with an extensive evidence base and extensive experience of use in Russian clinical practice.
Metformin during pregnancy: what results should you expect?
Relevance
In recent years, the prevalence of type 2 diabetes mellitus (DM) in pregnant women has increased worldwide.
Insulin therapy is the standard treatment for diabetes during pregnancy. Increasing insulin resistance during pregnancy requires increasing insulin doses, which in turn is associated with weight gain in patients, a large number of painful injections, high drug costs and low compliance.
At the moment, there is insufficient data on the characteristics of type 2 diabetes treatment with metformin in pregnant women.
Scientists from Canada studied the effects of metformin in the context of possible reduction in the risk of adverse outcomes for the mother and fetus.
Materials and methods
The MiTy randomized controlled trial was conducted.
The study results were presented at the professional conference Diabetes UK: Online Series on November 17 and recently published in The Lancet Diabetes & Endocrinology.
We studied 502 women from 29 centers in Canada and Australia who were diagnosed with type 2 diabetes before pregnancy or during pregnancy before 20 weeks' gestation.
Patients were randomized to receive metformin 1 g twice daily or placebo in addition to their usual insulin regimen from 6 to 28 weeks of pregnancy.
results
- Type 2 diabetes was diagnosed before pregnancy in 83% of women in the metformin group and in 90% of women receiving placebo.
- The average level of glycated hemoglobin (HbA1C) at randomization was 47 mmol/mol (6.5%) in both groups.
- The mean age of the mothers was approximately 35 years and the mean gestational age was 16 weeks.
- The average pre-pregnancy BMI was approximately 34 kg/m2.
- It is noteworthy that only 30% of the subjects belonged to the Caucasian race.
- There were no significant differences between groups for the composite primary outcome of miscarriage, preterm birth, birth trauma, respiratory distress syndrome, neonatal hypoglycemia, or neonatal intensive care unit admission for more than 24 hours (P = 0.86).
- Women in the metformin group had significantly less total weight gain during pregnancy than women in the placebo group, -1.8 kg (P < 0.0001).
- Pregnant women in the metformin group also had significantly lower HbA1C levels during pregnancy, 41 mmol/mol (5.9%) compared with 43.2 mmol/mol (6.1%) in those receiving placebo (P = 0.015); they required fewer insulin doses, 1.1 versus 1.5 units/kg/day (P < 0.0001), resulting in a reduction in daily dose of nearly 44 units.
- Among women receiving metformin, doctors were less likely to perform a cesarean section at birth: 53.4% versus 62.7% in the placebo group (P = 0.03).
- There were no significant differences in gestational hypertension or preeclampsia in the compared groups.
- The most common adverse events occurred from the gastrointestinal tract: 27.3% in patients in the metformin group and 22.3% in those receiving placebo.
- There were no significant differences between groups in rates of miscarriage (P = 0.81), preterm birth (P = 0.16), birth injury (P = 0.37), respiratory distress syndrome (P = 0.49), and congenital anomalies (P = 0.16).
- The mean weight of infants born to mothers receiving metformin was lower, 3.2 kg, compared with 3.4 kg for those born to mothers in the placebo group (P = 0.002).
- Women receiving metformin were also less likely to have a baby with a birth weight of 4 kg or more, 12.1% versus 19.2%; relative risk 0.65 (P = 0.046), or a baby who was extremely large for gestational age, 8.6% vs. 14.8%; relative risk 0.58 (P = 0.046).
- Notably, metformin was also associated with an increased risk of small gestational age births by 12.9% compared with 6.6% in the placebo group; relative risk 1.96 (P = 0.03).
Conclusion
Metformin as a therapy for type 2 diabetes during pregnancy, in addition to controlling glycemic levels, has the following pleiotropic effects: weight loss, reduction in the required dose of insulin, and reduction in the risk of developing a fetus that is large for gestational age.
Intrauterine growth retardation, smoking, serious kidney pathology, and low body mass index (BMI) are risk factors for the development of a small-for-gestational-age fetus while taking metformin in pregnant women with type 2 diabetes.
Metformin inhibits the mTOR pathway, which is the primary sensor of nutrients in the placenta and may impair fetal growth.
The research team has launched the MiTy Kids study, which will follow children born to mothers treated with metformin to determine whether metformin during pregnancy is associated with reduced obesity and improved insulin resistance in children at 2 years of age.
If metformin was started before pregnancy due to fertility problems, its discontinuation is recommended immediately after pregnancy or during the first trimester.
medscape.com/viewarticle/941337
Metformin Long - start of therapy for type 2 diabetes mellitus
One of the most common diseases in modern society is diabetes mellitus (DM). The medical and social significance of diabetes is determined by the steady increase in the number of patients, the high risk of macro- and microvascular complications, as well as the impact of the disease on the quality and life expectancy of patients. A similar global trend of steady growth in the number of people with diabetes mellitus also occurs in the Republic of Belarus. Over the past 10 years, the number of such patients in Belarus has increased from 150 thousand to 336 thousand people, 94% of whom suffer from type 2 diabetes (data as of 01/01/2019). And every year there are more patients with type 2 diabetes in the country. Currently, the drug metformin occupies a central place in all current recommendations for the management of type 2 diabetes.
Thus, the 2022 consensus algorithm of the American and European Diabetes Associations (ADA/EASD) strongly recommends the use of metformin as a first-line agent (in the absence of contraindications or intolerance), immediately upon diagnosis of type 2 diabetes, in addition to image correction life (dietary recommendations and exercise). The preference given to this drug is not accidental: the effectiveness and safety of metformin have a huge evidence base, its cost is low, the drug reduces the risk of developing cardiovascular complications, helps reduce body weight, and the risk of hypoglycemia when taking it is minimal. In 2022, the American Diabetes Association released new recommendations that metformin remains the preferred drug for initiating glucose-lowering therapy in patients with type 2 diabetes (Evidence Level A). However, unfortunately, the tolerability of metformin is limited by side effects from the gastrointestinal tract, which, according to some data, develop in almost 25% of patients, which leads to discontinuation of the drug in 5-10% of patients. In addition, splitting up metformin intake throughout the day and a large number of tablets, if necessary, to take other drugs to treat concomitant diseases, creates additional inconvenience for patients. Insufficiently good tolerability from the gastrointestinal tract and the need to take it more than once a day leads to decreased adherence to metformin therapy in some patients. The new extended-release dosage form of metformin has been designed to provide sustained release of the drug to improve tolerability and ensure prolonged action of the drug. This dosage form helps slow down the transit of the tablet from the stomach through the pylorus, thereby significantly increasing the time the drug remains in the stomach. In vitro studies have shown that 90% of metformin in the conventional dosage form is released within 30 minutes. In contrast, extended-release metformin releases 90% of the drug within 10 hours, relatively independent of gastrointestinal pH and motility. Thus, the dosage form of Metformin Long provides a slower, smoother and longer delivery of the drug without an initial rapid increase in the concentration of the drug in the plasma. When prescribed in the evening with food, due to its dosage form, Metformin Long acts synchronously with natural physiological processes, when gastrointestinal emptying slows down at night. This leads to prolonged absorption of metformin and justifies the administration once a day.
However, patients should be aware and warned that the inactive portion of the metformin extended-release tablet is excreted unchanged. Otherwise, this may lead to erroneous conclusions and unmotivated refusal to use this dosage form. The hypoglycemic effect of modified-release metformin is comparable to that of immediate-release metformin. Thus, modified-release metformin, having all the advantages of the conventional form of the drug, is devoid of a significant part of its disadvantages: taking extended-release metformin once a day significantly simplifies the treatment regimen and, together with a decrease in the frequency of side effects, increases patient adherence to treatment. Metformin Long, the first long-acting generic metformin in the Republic of Belarus, is produced by the Borisov Medical Preparations Plant Open Joint Stock Company in dosages of 500 mg, 750 mg and 1000 mg. The indication for the use of drugs Metformin Long 500, 750, 1000 is type 2 diabetes mellitus in adults (especially in overweight patients) with ineffective diet therapy and exercise, as monotherapy or in combination with other oral hypoglycemic agents or together with insulin . When used as monotherapy or combination therapy in combination with other oral hypoglycemic agents, Metformin Long is used as follows:
- The recommended initial dose is 1 tablet of Metformin Long 500 per day.
- After 10-15 days of treatment, the dose must be adjusted in accordance with the results of measurements of glucose levels in the blood serum. Slowly increasing the dose helps reduce gastrointestinal side effects. The maximum recommended dose is 4 Metformin Long 500 tablets per day (2000 mg).
- It is recommended to take the dose of the drug once a day with meals in the evening, increasing by 500 mg every 10-15 days to a maximum dose of 2000 mg. If, when using Metformin Long at a maximum dose of 2000 mg 1 time per day, the required level of glycemia cannot be achieved, the dose taken can be divided into 2 doses (morning and evening during meals). If the required glycemic level is not achieved after this, metformin immediate-release tablets can be used at the maximum recommended dose of 3000 mg per day.
- For patients already taking metformin, the starting dose of Metformin Long should be equivalent to the immediate-release tablet dose. For patients taking metformin at a dose above 2000 mg, switching to Metformin Long is not recommended.
- If you switch to Metformin Long, you must stop taking the other antidiabetic drug.
- Metformin Long 750 and Metformin Long 1000 are intended for patients already receiving metformin immediate-release tablets.
- Doses of Metformin Long 750 or Metformin Long 1000 should be equivalent to the daily dose of metformin immediate-release tablets with a maximum dose of 1500 mg or 2000 mg, respectively, taken in the evening with meals.
In combination therapy in combination with insulin, Metformin Long is used as follows:
- To achieve better control of blood glucose levels, metformin and insulin can be used in combination therapy. Typically, the initial dose of Metformin Long 500 is 1 tablet per day with meals in the evening; the dose of insulin must be adjusted in accordance with the results of measuring blood glucose levels.
- For patients already receiving metformin and insulin in combination therapy, the dose of Metformin Long 750 or Metformin Long 1000 should be equivalent to the daily dose of metformin with a maximum dose of 1500 mg or 2000 mg, respectively, in the evening with meals, while the insulin dose is adjusted to based on blood glucose measurements.
- For elderly patients and patients with reduced renal function, the dose is adjusted based on assessment of renal function, which should be carried out regularly.
- Due to the lack of data on use, Metformin Long should not be used in children under 18 years of age.
- Medicines Metformin Long 500, Metformin Long 750, Metformin Long 1000 are available with a doctor's prescription.
Rules for writing a prescription
Metformin is dispensed at pharmacies, subject to a prescription. The Latin prescription for Metformin must be written correctly. If there are errors in the document, it will be rejected by the pharmacist.
In Latin, the prescription for Metformin allows you to prescribe the form of the drug, dosage, and rules of administration. The document begins with the word Recipe (take). It is written on a new line. The word Recipe can be abbreviated as follows: Rp. This designation must be followed by a period and a colon (Rp.:).
After the word Recipe, write the name of the drug in Latin, the dosage and form of the medicine. In this case, the doctor will write the following: “Tabulettas Metformini 1.0.” The word Tabulettas can be abbreviated as Tab.
After describing the dose, form and name of the medicine, the doctor prescribes in the second line of the prescription the number of forms that the pharmacist must dispense. Example: “Da tales doses numĕro 30” - give out such doses in number 30. This sentence can be shortened as follows: D. td N 30.
After the number of doses, the doctor prescribes a signature. The signature reveals the rules for using the drug. The third line of the document begins with the word Signa (indicate). It is abbreviated as follows: S. In the signature, the doctor writes how many tablets the patient should take, and how often. This concludes the writing of the prescription. After the signature there is a sign - #. It indicates that the recipe is complete.
In Latin, the prescription for Metformin is written as follows:
- Rp.: Tab. Metformini 1.0
- DTD N 30
- S. 1 tablet 2 times a day with meals
- #
Side effects of metformin
- With long-term use of metformin, a decrease in the absorption of vitamin B12 may be observed. If megablastic anemia is detected, additional intramuscular administration of vitamin B12 is necessary.
- Quite often, problems with the gastrointestinal tract occur (nausea, vomiting, diarrhea, bloating, abdominal pain, changes in taste, loss of appetite). In this case, metformin should be taken with food to reduce gastrointestinal irritation.
- With long-term use, as well as when taking metformin with large doses of alcohol, lactic acidosis may occur - a high level of lactic acid in the blood, which can threaten the patient's life. Occurs more often with an overdose of metformin and in patients with renal failure.
- Very rarely, skin reactions - erythema, rash, itchy dermatoses.
- Very rarely, liver dysfunction, hepatitis, which disappear when the drug is discontinued.
Metformin is used only as prescribed by a doctor and is available only with a prescription.
Known mechanisms of action of metformin
The most important effect of metformin is to suppress the production of glucose by the liver.
Metformin activates the release of the liver enzyme AMPK, which is responsible for the metabolism of glucose and fat. This activation leads to an inhibitory effect on hepatic glucose production. That is, excess glucose is not produced due to metformin.
In addition, metformin increases sensitivity to its own insulin and increases peripheral glucose uptake (with the help of insulin, glucose is delivered to all cells of the body and becomes a source of energy), increases the oxidation of fatty acids, and reduces the absorption of glucose in the gastrointestinal tract.
The delay of glucose absorption in the gastrointestinal tract by metformin allows one to maintain lower blood glucose levels after meals, as well as increase the sensitivity of target cells to their own insulin. This property of metformin allows it to be used for prediabetes - to prevent diabetes if you are prone to it.
After oral administration, metformin is absorbed from the gastrointestinal tract, its active effect begins after 2.5 hours. Metformin is excreted by the kidneys after about 9–12 hours. It should be noted that metformin can accumulate in the liver, kidneys and muscles.
The use of metformin begins with taking 500–850 mg 2–3 times a day during or after meals. A further gradual increase in the dose is possible depending on the results of blood glucose concentration tests.
The maintenance dose of metformin is usually 1500–2000 mg/day.
To reduce side effects from the gastrointestinal tract, the daily dose is divided into 2-3 doses. The maximum daily dose is 3000 mg/day, divided into 3 doses.
The original drug of metformin is the French Glucophage.
Generics of Glucophage: Metformin from Ozon (Russia), Siofor, etc.
Still, to reduce the side effects of metformin (gastrointestinal disorders) and improve the quality of life of patients with type 2 diabetes mellitus, long-acting metformin called Glucophage Long with delayed absorption of active metformin was developed and released in France. Glucophage Long can be taken once a day, which, of course, is much more convenient for patients.
Long-acting metformin is absorbed in the upper gastrointestinal tract.
Characteristics of the drug
Metformin is considered a hypoglycemic drug that is often prescribed to diabetic patients. The drug can be purchased at pharmacies in tablet form. The product has an average cost of 93 – 465 rubles. Analogues of the drug are Siofor, Metformin - Teva. The active ingredient of the drug is metformin hydrochloride. Tablet forms can have the following dosages: 500 mg, 850 mg, 1000 mg.
The active substance inhibits the process of gluconeogenesis in the liver tissue and reduces the concentration of fatty acids. The medicine prevents the oxidation of fat molecules. The product facilitates the absorption of glucose molecules. Metformin is not able to change the amount of insulin in the blood. The drug promotes weight loss.
Metformin is permitted for use in diabetic patients. The medicine can be used in adult patients and children over 10 years of age. The drug can be used simultaneously with insulin or as monotherapy.
Restrictions on prescribing the medicine:
- metabolic acidosis;
- coma, precoma in patients with diabetes;
- serious impairment of the kidneys and liver;
- severe infectious pathology;
- hypoxic conditions (heart pathologies, respiratory dysfunction);
- intravenous administration of drugs containing iodine for X-ray examinations and computed tomography;
- poisoning from alcohol-containing drinks and medications;
- patients under 10 years of age;
- allergy to the components of the drug.
The medicine should be used carefully in patients with serious pathologies of the kidneys and liver, patients over 60 years of age. The drug should be used carefully in patients 10-12 years of age.
The medicine is not prescribed during pregnancy and breastfeeding. In this group of patients, the effect of the drug on the body has not been fully studied. The drug should not be combined with alcohol-containing drugs and drinks, glucocorticosteroid hormones, diuretics, antihypertensive drugs and other drugs.
A serious complication of Metformin overdose is lactic acidosis. High dosages can also provoke a hypoglycemic state. Lactic acidosis and hypoglycemia can lead to a deterioration in the patient's condition, which is life-threatening.
Is Metformin a cure for everything? Yes, but no
We are here, in the editorial office of “XX2 Century”
, we love metformin very much and talk about it at every opportunity. Today it suppresses the growth of cancer cells, tomorrow it fights inflammation, the day after tomorrow it helps you lose weight, and next week it completely prolongs life. Readers might have the impression that this medicine overcomes any disease. Unfortunately, this is not entirely true and almost every piece of good news comes with a “yes, but.” So we decided to write about metformin again - this time a long text that will explain to those who missed everything where this drug came from, what it treats and how successful it is.
Story
It all started with grass. Goat's rue, aka goat's rue, aka Italian ferret, aka, scientifically, Galega officinalis
- a perennial herbaceous plant. In medieval Europe, it was used to treat frequent urination - one of the symptoms of diabetes - and some other diseases. It is difficult to say how long goat's rue has been used in folk medicine, but it is known that the famous English aesculapian Nicholas Culpeper mentioned it back in 1652 in the book “The English Physician”.
At the end of the 19th century, scientists took a closer look at goat's rue and found that it contains large amounts of guanidine (this colorless crystalline substance was first synthesized in 1861). In 1918, during experiments on rabbits, scientists showed that guanidine lowers blood glucose levels. But the compound turned out to be too toxic to treat people, so scientists began experimenting with guanidine derivatives. In 1922, Emil Alphonse Werner and James Bell synthesized N, N-dimethylguanidine to obtain dimethylbiguanidine, known to us as metformin, and seven years later the German scientist Karl Slotta tested it on animals . There were other drugs based on guanidine - galegin (isoamylene-diguanidine), biguanides Syntalin A and B. Synthalins were even used in clinical practice for some time, but after the industrial production of insulin began (in 1923 by Eli Lilly and Company
started selling it under the name “Iletin”), they forgot about guanidine derivatives.
Galega officinalis
or goat's rue is the grandfather of the most popular antidiabetic drug.
In 1949, metformin came into the hands of Philippine infectious disease specialist Eusebio Garcia. He called the substance “flumamine” and used it to treat influenza and malaria. A year later, in the article Fluamine, a new synthetic analgesic and antiflu drug, Garcia said that a single injection of the drug relieved the headaches of thirty patients and completely cured them in 24 hours. The doctor did not know the exact mechanism of action, and suggested that flumamine lowers the concentration of sugar in the blood, but did not provide any evidence.
These speculations were enough to interest another physician, the Frenchman Jean Stern, a specialist in diabetology. He conducted experiments on dogs, rats and rabbits and found that six months of treatment had no effect on their development or liver function. Even during the autopsy, no anomalies were found. Stern conducted clinical trials on humans, called the drug a “glucophage” (“sugar eater,” similar to a bacteriophage) and began treating diabetics with it.
French doctor Jean Stern and the hospital where he studied the properties of metformin.
Metformin almost immediately had competitors - the more powerful phenoformin and buformin. But these drugs caused lactic acidosis - a dangerous condition that is accompanied by depression of the central nervous system, impaired breathing, cardiovascular function and urination. Therefore, by the end of the 70s they were no longer used in most countries. And that’s when metformin became the main alternative to insulin. It was sold in France and the UK in the late 50s, in Canada in the 70s, and only entered the American market after approval by the Food and Drug Administration (FDA) in 1994 year. There he quickly became a “bestseller”
What do we know about metformin
Metformin is now considered a “first-line drug” for the treatment of type 2 diabetes. Its main advantage is that it practically does not cause hypoglycemia, which distinguishes it from insulin and another class of glucose-lowering drugs - sulfonylurea derivatives. Metformin reduces blood glucose concentrations by inhibiting its production in the liver, while sulfonylureas increase the release of insulin from beta cells in the pancreas. In addition, it does not promote weight gain. Of course, it also has side effects, unpleasant, but not fatal: the most common are gastrointestinal disorders, in particular nausea, vomiting, flatulence and diarrhea. And reducing appetite is even beneficial.
Much of what we know about the health effects of metformin comes from clinical studies. This drug was originally developed to lower blood sugar, and was studied, of course, primarily in the context of diabetes. In order to talk about the advantages and disadvantages of metformin, you need to talk at least a little about how doctors obtained this information - otherwise the story will turn into “scientists have proven it.” Here are some of the most significant clinical trials on type 2 diabetes:
- The University Group Diabetes Program, UGDP (University Group Diabetes Program)
- United Kingdom Prospective Diabetes Study, UKPDS (British Prospective Diabetes Study)
- Diabetes Prevention Program, DPP (Diabetes Prevention Program)
The UGDP was the first randomized clinical trial focused on diabetes. It involved 1,027 people, the study lasted 21 years, from 1960 to 1981, and the first results were published in 1970. Scientists wanted to find out which medicine is most effective in preventing the development of cardiovascular complications. The clinical trial was subject to fierce criticism, including due to errors in randomization. However, the FDA found no reason to disbelieve its findings. Participants were not taking metformin, but the UGDP declared another drug in the same class, phenformin, ineffective, and as a result, the “similar” drug was not approved for use in the United States. It was thanks to the UGDP that the drug's entry into the market in this country was delayed for many years. UKPDS was the largest clinical study at that time - it included 5102 patients with type 2 diabetes. The test lasted 20 years, from 1977 to 1997. UKPDS was meant to answer the question: can intensive blood glucose control prevent complications, and what is the best medicine to do this? Participants were taking first-generation sulfonylureas, insulin, or following a diet. After the publication of the results, doctors began to recommend metformin more often.
3,234 people participated in the DPP The goal of the study was to find the most effective way to prevent type 2 diabetes in people with prediabetes. To do this, one group was given diet, exercise and lifestyle changes, another was given metformin, and the third was given a placebo. After the clinical trial, they conducted another one - the Diabetes Prevention Program Outcomes Study, DPPOS, or, in Russian, “Diabetes Prevention Program: Study of the Outcomes.” Doctors studied the health status of DPP participants after 15 years.
During the UKPDS, only overweight patients received metformin. The results showed that the drug reduced the risk of death from diabetes complications by 42% and all-cause mortality by 36%. A good result, but not so impressive considering that the drug was compared to a regular diet. The risk of cardiovascular complications in patients on metformin, insulin and sulfonylureas was practically no different. When paired with urea derivatives, the drug even increased mortality. But, unlike other drugs, metformin did not promote weight gain and was less likely to cause hypoglycemia. Therefore, scientists have proposed no less than metformin as a first-line drug in the treatment of obese patients. This marked the beginning of his popularity.
DPP/DPPOS showed that taking metformin could reduce the risk of developing diabetes in people with prediabetes by 31%. But it’s better to change your lifestyle - in this case, the incidence is reduced by 58%. Good old exercise and diet turned out to be almost 2 times more effective. But the drug had another advantage - metformin helped to lose weight. People with prediabetes who took the drug lost an average of two kilograms. The effect lasted as long as the study participants took the pills, and the medicine was well tolerated.
Of course, there have been other studies of metformin and even meta-analyses of these studies. However, we still do not know everything about the advantages and disadvantages of this drug. For example, scientists are still trying to figure out how metformin affects the development of cardiovascular diseases and mortality - data on this matter is contradictory. A 2011 review of 30 papers found that a diabetes drug did neither significant harm nor significant benefit to the heart, and only performed well when compared to a placebo or no treatment at all. A 2016 paper that analyzed 300 studies found that there was no difference at all between nine classes of glucose-lowering drugs in terms of mortality and cardiovascular disease. However, the authors of the analysis acknowledge that the selected articles had a high risk of bias - more than half of the publications presented information selectively, and sponsors participated in the work on them. On the other hand, a recent study based on 17 publications shows that in patients with chronic kidney disease, congestive heart failure and chronic liver failure, metformin does reduce mortality.
New application
In Russia and the USA, metformin is approved only for the treatment of type 2 diabetes, but they are also trying to treat other diseases. And the more that becomes known about the mechanisms of action and the effect of the drug on the body, the more actively they are looking for new uses for it.
It has long been known that taking metformin is accompanied by weight loss. DDP and DDPOS did not open doctors' eyes, but only confirmed previous observations. Therefore, doctors tried to treat healthy obese people with metformin. One of the first such studies was published in 1970 in the Lancet
. Scientists compared the effectiveness of metformin and fenfluramine on the example of 34 women aged 22-59 years. After 8 weeks of therapy, they concluded that fenfluramine worked better and had fewer side effects.
Work from 1998 and 2001 rehabilitated the glucose-lowering drug and showed that it reduced weight in non-diabetics, but a later meta-analysis came out that called these results into question. The scientists selected 57 studies and excluded 48 of them because they did not meet clinical trial standards. There were only 9 left - and after analysis it became clear that there was not enough data on the effectiveness of metformin. Three years later, another review came out and confirmed the results of the previous one. Metformin seemed to reduce weight by 3-9 kilograms, but the sample of such studies was small, the duration was short, and the design was “weak.” In addition, in addition to taking the medicine, the participants did physical exercise - try to figure out what exactly helped them lose weight. Several longer trials without these shortcomings showed only minor weight loss.
Four years ago, the results of perhaps the largest clinical study devoted to the treatment of obesity in non-diabetics were published. If in previous works we were talking about three to four dozen people, now there were 200 volunteers. 20% were unable to lose weight at all, and 9 people gained it. The remaining study participants lost an average of 5% of their body weight, and the drug helped people with impaired insulin sensitivity the most. But there was also a methodological pitfall in this study: there was no randomization of the control group.
Overweight children and adolescents are also treated with metformin, but not particularly successfully. In 2016, Cochrane, an international NGO that studies the effectiveness of health technologies, presented a systematic review and found that not only metformin, but also other drugs are ineffective in this population. And the quality of the available data leaves much to be desired. In general, doctors admit that it is too early to recommend a diabetic drug for the treatment of obesity for both children and adults. The problem is still solved mainly by diet and exercise.
Another use of metformin is in the treatment of polycystic ovary syndrome (PCOS). PCOS is a condition in which women's levels of male hormones (androgens) increase, the functioning of the ovaries is disrupted and the menstrual cycle is disrupted. As a result, ovulation does not occur, and it becomes difficult (although not always impossible) to get pregnant. Many patients experience excess hair growth on the face and body, acne appears, and about half gain excess weight. It was not possible to fully understand the causes of PCOS, and it is not possible to cure it once and for all. But it is already known that the disease is associated with tissue insensitivity to insulin, and women with this syndrome have an increased risk of developing type 2 diabetes. Metformin, which fights insulin resistance, comes in handy here.
But it's not that simple. In 1994, the drug worked well - then Venezuelan scientists conducted a study with the participation of 29 women, 7 of whom restored their menstrual cycle, and three spontaneously became pregnant. “Many, if not all, of the metabolic disorders of PCOS can be reversed with metformin,” the authors wrote. True, they immediately stipulated that they did not perform randomization and did not use a placebo for comparison. However, larger and more sophisticated clinical trials in 2006-2007 did not confirm the optimistic assumptions. Metformin did not stimulate ovulation particularly effectively and was inferior to clomiphene (trade name Clostilbegit).
According to the clinical guidelines of the International Endocrine Society, the diabetic drug helps with metabolic disorders and irregular periods, but has limited or no effectiveness in treating infertility, acne and excess hair growth. Participants in a seminar organized by the American Society for Reproductive Medicine came to a similar conclusion. They recommended metformin only for women with impaired glucose tolerance. But a 2014 Cochrane review says the drug is more effective than placebo and increases the chance of getting pregnant. True, the quality of the evidence base of the studies was considered low, and the likelihood of error was considered high.
And metformin, theoretically, can help fight cancer. But to what extent these hopes are justified is still unknown. They began to take a serious look at the medicine from this side only 12 years ago - an insignificant period if you remember how long it lasted, for example, UKPDS. In 2005, Josie Evans and her colleagues from the University of Dundee reported that taking metformin reduced the incidence of cancer among diabetics by 23%. They came to these conclusions after analyzing data from the DARTS electronic medical database.
The publication inspired other doctors, and they began to double-check their colleagues’ findings using other medical registries. Gradually, more and more studies confirmed the benefits of metformin. Reviews of scientific papers appeared that showed a decrease in incidence at the level of 31-34%. Then - experiments on cell cultures and mice. It turned out that in rodents, under the influence of a diabetic drug, the growth of tumors slows down by as much as 50% - however, the drug was fed to them in doses exceeding human doses.
Why can’t we please readers and say that we have finally found a new effective cure for cancer? For two reasons. Firstly, there is no abstract “cancer” - it is a collection of different oncological diseases. How effectively metformin combats each of these needs to be tested in clinical trials, a process that is far from complete. Second, the reliability of the data that generated early enthusiasm has been called into question. In two dozen papers, errors were found that could distort the results. And where they didn’t find it, they didn’t find a connection between metformin and cancer incidence. In general, more time is needed, more research is needed.
Metformin and aging
If metformin is mentioned in the media, the article is probably talking about life extension. Gerontologists look to the old drug with hope, and they have good reason for this. Firstly, in recent years, scientists have begun to actively study the molecular and genetic mechanisms of aging. Research shows that calorie restriction increases the lifespan of mice and rats by about 30 to 40%. According to an article published this year, such “therapeutic fasting” also prolongs the life of primates. “What does metformin have to do with it?” you ask. According to some scientists, metformin causes approximately the same changes in the body as calorie restriction: it increases sensitivity to insulin, lowers cholesterol, and improves physical condition. And gene expression in rodents fed metformin resembles that in animals on a low-calorie diet.
So scientists set out to test the drug’s effect on model organisms. Caenorhabditis elegans worms treated with metformin lived 18-36% (depending on the dose) longer than their relatives from the control group. Mice - by 5%. By the way, similar experiments were carried out here in Russia, at the Oncology Research Institute named after. N. N. Petrova. Scientists fed metformin not to ordinary rats, but to a breed created specifically for studying hypertension and cardiovascular diseases. In such animals, the average life expectancy increased by 37.8%. Gerontologists even got to crickets: in the species Acheta domesticus, after treatment with an antidiabetic drug, the maximum life expectancy was 138% compared to the control group.
The next logical step would be to conduct human trials. The results of one such study have already been published. Scientists analyzed data from 180,000 people: 78,000 had diabetes and took metformin, 12,000 took sulfonylureas, and 90,500 were healthy. This information was obtained not from a double-blind, placebo-controlled trial, but from the Clinical Practice Research Datalink. The results showed that diabetics on metformin live 15% longer than healthy people. The media wrote about a drug that can prolong life, but some scientists' work entitled “Can people with type 2 diabetes live longer than healthy people?” I wasn't impressed at all. Here's what Kevin McConway, a professor of applied statistics at the British Open University, said about it:
“The title of the article is misleading because someone who reads it may misunderstand it - in fact, this study cannot answer the question asked for reasons I will give below, and it seems as if doctors have reason to recommend metformin for healthy people. But this is not what the study is about.
In the press release, Craig Curry says, “Once a person develops diabetes, their life expectancy is shortened by an average of 8 years,” and goes on to explain why. If diabetics live so much shorter than healthy people, how can they “live longer than those who don't have diabetes,” as the article and press release headlines say?
That's because the study focused on a period of time when diabetic patients were receiving metformin as first-line therapy (this group was also compared with those who received sulfonylureas as first-line therapy). At some point, many patients will be transferred to second-line therapy because their diabetes or its symptoms have worsened. But at this point the research simply ends.
So the quote about reducing life expectancy in patients with type 2 diabetes by 8 years is talking about the patient's entire life after diagnosis, including the period when they are on more aggressive second-line therapy. But the study only takes into account the period of time before a change in treatment regimen. This won’t fit into a succinct headline, but it’s important to note that it’s not all that simple.
But if the survival rate of diabetics taking metformin is significantly higher than that of healthy people, albeit for a limited period of time, doesn't that mean that people who don't have diabetes should take metformin to live longer? No, it doesn't mean that. This apparent difference may be due to something other than metformin. […]
The difference in survival between diabetics on metformin and controls was statistically significant, but essentially quite small and likely within the range that can be explained by residual confounding (the influence of other variables not taken into account in the analysis).”
In addition, it would be remiss not to mention that the study was funded by pharmaceutical companies AstraZeneca
and
Bristol-Myers Squibb
.
Employees of these firms had access to the study data, although the article says they had no influence on the analysis, peer review or publication process. And the scientists themselves are quite familiar with the pharmaceutical industry. Five worked for Pharmatelligence
, a research consulting company that receives grants from drug manufacturers.
Another was an employee of Bristol-Myers Squibb
. This in itself does not make the results unreliable, but, you see, it is somewhat alarming. Especially considering the fact that both sponsoring companies produce metformin.
So, without full-fledged clinical trials, this issue cannot be understood. One of the first studies on the connection between metformin and aging was to be the Metformin in Longevity Study. But since its launch in 2014, no information has appeared about it. Preliminary results were supposed to be obtained two years ago, but since then not a single publication has been published.
Another clinical trial is approaching - Targeting age with Metformin (TAME). This double-blind, randomized, placebo-controlled study is the gold standard, just the way we like it. It will be attended by 3,000 people aged 65 to 79 years. TAME is quite unusual for several reasons. Firstly, it is aimed at studying something that does not seem to exist. Aging, from a medical point of view, is not a disease. There is also no generally accepted biomarker by which one could understand whether this process is slowing down or not. Therefore, the goal of the study is to determine whether metformin can slow down the development of age-related diseases: cardiovascular, neurological, oncological, and so on.
Scientists hope they can set a precedent so that in the future the FDA will recognize aging as an “indication,” a condition that can be treated. "I think the FDA would be more likely to accept something called 'comorbidities' than something called 'aging,'" said Nir Barzilai, the team's chief scientific officer. - Even in our... in my opinion, old age is not a disease. It's human nature, you know! We are born, we die, and in between we grow old... I mean, “I don’t care what you call it, as long as I can delay it.”
Another unusual detail is that the study is not sponsored by pharmaceutical companies or the government. Funds are raised by the American Federation for Aging Research, and anyone can donate. According to Barzilai, conducting clinical trials will cost "$50 million, give or take 20 million." The results will not come soon: it will take some time to raise money, the research itself will last 3-4.5 years, plus time for processing and publishing the data. But when it comes to prolonging life, you can wait.
You might be interested in:
Metformin may be helpful for autism.