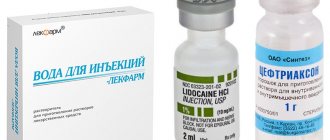
Composition and dosage form
The drug is produced in the form of a lyophilized concentrate - a dense mass of greenish-yellow color. In some cases - in the form of large tablets sold together with a solvent. The drug is intended for parenteral administration: making intramuscular and intravenous injections. The cardboard packaging of Artoxan contains 3 sealed glass bottles of lyophilisate and ampoules with water.
The active substance is tenoxicam, which has a complex antipyretic, analgesic and antiplatelet effect. A dose of Artoxan contains 20 mg. tenoxicam, auxiliary and stabilizing components.
Mechanism of action
Artoxan inhibits the production of prostaglandins and the accumulation of enzymes in lesions, including in the cartilaginous elements of the skeleton. The drug has a cumulative effect. A pronounced anti-inflammatory and analgesic effect develops 5–7 days after the first injection. Therefore, the drug is used for joint pathologies.
In addition to eliminating the symptoms of inflammation and pain, the drug reduces platelet aggregation, preventing the formation of blood clots and thrombi. When the active substance enters the blood, it quickly penetrates into all tissues and fluids and interacts with albumin almost 100%. Metabolism occurs in liver cells. Excretion of the drug is regulated by the intestines and kidneys.
When is Artoxan needed?
The scope of application of non-steroidal anti-inflammatory drug injections is pathology of the musculoskeletal system: the pain syndrome they cause. Injections are prescribed:
- for osteoarthritis;
- degenerative processes in the area of hard tissues of the spine and joints;
- exacerbations of osteochondrosis;
- for rheumatoid arthritis;
- for gout;
- tendovaginitis, bursitis, consequences of mechanical injuries to joints and ligaments.
Artoxan is also used to relieve pain of various origins: headaches, dental pain, neuralgic attacks, migraines, pathologically painful periods. And also for burns and soft tissue injuries.
ARTOXAN (lyophilisate)
I thought that from time to time the knee would still become inflamed (especially in the fall and spring) and I just needed to get injections.
Surprisingly, dikloberl helped. How long did it take for the knee to become inflamed again? Again the same injections. So 10 years passed. But every year my inflammations became more frequent. And now less than a month has passed and everything is new. More pain (not aching) at night was added. At the slightest load. I completely forgot about heels. In general, the injections stopped working for me, even the most powerful pain-relieving ointments. I couldn’t stand it and went to a paid doctor. I did an ultrasound. It turned out that I don’t have any arthritis, it’s a meniscal cyst that has been growing all these years. In addition, this cyst caused the initial stage of arthrosis. To be honest, I was in shock. The doctor said that in order to rule out any inflammation and 100% confirm the cyst, it is necessary to undergo treatment and again for a control ultrasound. And then there will be further actions to eliminate the cause. For this purpose, he prescribed a course of Mucosata and Artoxan. 2. ARTOXAN. This is a drug from the group of non-steroidal anti-inflammatory drugs. It helps eliminate the inflammatory process. It also has an analgesic and antipyretic effect. Indications for use: - inflammatory-degenerative. diseases of the musculoskeletal system that are accompanied by pain (rheumatoid arthritis, gouty arthritis, ankylosing spondylitis); — infectious nonspecific polyarthritis; - osteoarthritis, osteochondrosis; — tendonitis, bursitis, myositis, periarthritis; - neuralgia, myalgia, ischalgia, lumbago; - injuries, muscle strains, burns. As you can see, there is a lot of evidence. There are no less side effects. All this can be read in the instructions.
Contraindications One package contains 3 ampoules with medicinal powder and 3 ampoules with water for injection. Injects once a day. Treatment usually does not exceed 7 days. But in severe cases it can be up to 14 days. The price in Belarus for 1 package is almost 20 rubles or 10 dollars.
3. RESULT. The injections themselves are a little painful. But if we compare it with antibiotics, then this is “heaven and earth”; compared to them, artoxan is painless. In my case, it did not cure, but it relieved the pain a little.
*
!!! UPDATE !!!
I thought that it was only me that he did not cure anything, because the case was not suitable for the indications.
But just a couple of months ago, my aunt experienced joint pain. She has grade 1-2 osteoarthritis and a Becker cyst. The doctor prescribed artoxan injections. These injections did not play any role at all.
Then a sharp exacerbation began for my mother. They did an ultrasound and diagnosed: deforming osteoarthritis of 1-2 and 2 degrees, synovitis of the knee joint. Synovitis is inflammation. The doctor first prescribed one treatment. Did not help. Then artoxan (as many as 12 injections!). She didn't feel any better. Then he began to make a definition. injections in the leg, a little easier. It has only been a short time since the last injection into the joint, so it is too early to say whether another treatment helped her. But as I have seen, even according to the indications, the effectiveness of artoxan is questionable. At least for osteoarthritis and inflammatory joints there is NO effect!
How to use Artoxan: instructions
The drug does not affect the progress of the general disease; it is used only to reduce symptoms. The solution is prepared a few minutes before administration; the diluted medication cannot be stored.
- The contents of the bottle are combined with the liquid and thoroughly shaken several times until the mass is completely dissolved, becomes homogeneous, and yellowish in color.
- The needle in the syringe is replaced after mixing the drug.
- With an intramuscular injection, the drug is injected deep into the gluteal or other large muscle.
- For intravenous injection, the drug is injected as slowly as possible.
The frequency of Artoxan injections is 1 time per day, one dose of the drug. The duration of pain relief is no more than two days in a row. In the future, you need to use oral medications containing tenoxicam to avoid intoxication of the body.
Side effects
When using Artoxan, reactions from internal organs and systems are possible:
- from the digestive tract: heaviness in the abdomen, gastritis, nausea, abdominal cramps, heartburn, diarrhea;
- from the central nervous system: dizziness, asthenia, increased drowsiness, weakness, decreased mood, apathy, tinnitus, blurred vision;
- from the heart: tachycardia, arrhythmic manifestations;
- changes in blood count, high levels of liver tests, leukocytosis.
Skin symptoms are also likely: itching, erythema, rashes.
The drug is prescribed with caution to patients with low body weight, inflammatory diseases of the heart, kidneys, autoimmune pathologies, diabetes mellitus, and other chronic somatic disorders.
Artoxan
Tenoxicam has a high degree of binding to albumin and can, like all NSAIDs, enhance the anticoagulant effect of warfarin and other anticoagulants. It is recommended to monitor indicators when used together with anticoagulants and hypoglycemic drugs for oral administration, especially in the initial stages of using Artoxan.
No possible interaction with digoxin was noted.
As with other NSAIDs, it is recommended to use the drug with caution concomitantly with cyclosporine due to an increased risk of nephrotoxicity.
Concomitant use with quinolones may increase the risk of seizures.
Salicylates can displace tenoxicam from its binding to albumin and, accordingly, increase the clearance and volume of distribution of the drug. The simultaneous use of salicylates or two or more NSAIDs should be avoided (increased risk of gastrointestinal complications).
There is evidence that NSAIDs reduce lithium excretion. Therefore, patients receiving lithium therapy should monitor lithium blood concentrations more frequently.
NSAIDs may cause sodium, potassium, and fluid retention in the body, interfering with the action of natriuretic diuretics. This must be remembered when used together with such diuretics in patients with CHF and arterial hypertension.
It is recommended to use NSAIDs with caution in combination with methotrexate; NSAIDs reduce the elimination of methotrexate and may increase its toxicity.
NSAIDs should not be used within 8-12 hours after using mifepristone, because may reduce its effect.
It is necessary to take into account the increased risk of gastrointestinal bleeding when used together with corticosteroids.
Reduces the effectiveness of uricosuric drugs, enhances the effect of anticoagulants, fibrinolytics, side effects of mineralocorticosteroids and glucocorticosteroids, estrogens; reduces the effectiveness of antihypertensive drugs and diuretics.
Inducers of microsomal oxidation in the liver (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants) increase the production of hydroxylated active metabolites. Combined use with antiplatelet agents and selective serotonin reuptake inhibitors increases the risk of gastrointestinal bleeding.
Cardiac glycosides, when taken together with NSAIDs, can worsen heart failure, reduce glomerular filtration rate and increase plasma levels of cardiac glycosides.
No interaction has been identified when using tenoxicam with cimetidine. No clinically significant interaction has been identified during treatment with tenoxicam and penicillamine or parenteral gold.
The risk of nephrotoxicity increases when NSAIDs are used concomitantly with tacrolimus.
The risk of hematological toxicity increases when using NSAIDs with zidovudine.
Contraindications
It is necessary to stop using Artoxan:
- with high sensitivity of the body to ibuprofen, acetylsalicylic acid and other NSAIDs;
- allergic reactions to the components of the drug: skin itching, severe rashes;
- gastric ulcer, erosive lesions of the intestinal mucosa;
- for bronchial asthma in combination with other pathologies of the respiratory tract;
- in preparation for heart bypass surgery and during the recovery period after surgery;
- severe form of renal or liver failure;
- with heart failure (uncompensated);
- for pathologies of the hematopoietic system, low coagulability.
Artoxan is also contraindicated during pregnancy and for mothers who are breastfeeding, since its active substance penetrates the placenta and into milk.
The drug should not be used by patients under 18 years of age.
Artoxan lyophile d/i.v. and i.m. injection 0.02 mg No. 3 + solution
Compound
Active substance: tenoxicam 20 mg.
Excipients: mannitol - 80 mg, ascorbic acid - 0.4 mg, disodium edetate - 0.2 mg, trometamol - 3.3 mg, sodium hydroxide and hydrochloric acid - qs
Solvent composition (for 1 amp.): water for injection - 2 ml.
Pharmacokinetics
Suction
Absorption is fast and complete. Bioavailability 100%. Cmax in blood plasma is observed after 2 hours. The distinctive ability of tenoxicam is its long duration of action and long T1/2 - 72 hours.
Distribution
The drug is 99% bound to blood plasma proteins. Tenoxicam penetrates well into synovial fluid. Easily penetrates histohematic barriers.
Metabolism
Metabolized in the liver by hydroxylation to form 5-hydroxypyridyl.
Removal
1/3 is excreted through the intestines with bile, 2/3 is excreted by the kidneys in the form of inactive metabolites.
Indications for use
- Rheumatoid arthritis;
- osteoarthritis;
- ankylosing spondylitis;
- articular syndrome during exacerbation of gout;
- bursitis;
- tenosynovitis;
- pain syndrome (weak and moderate intensity): arthralgia, myalgia, neuralgia, migraine, toothache and headache, algomenorrhea;
- pain from injuries, burns.
The drug is intended for symptomatic therapy, reducing pain and inflammation at the time of use, and does not affect the progression of the disease.
Contraindications
- Hypersensitivity to the active substance or auxiliary components of the drug; there is a possibility of cross-sensitivity to acetylsalicylic acid, ibuprofen and other NSAIDs;
- erosive and ulcerative lesions of the stomach and duodenum in the acute phase;
- gastrointestinal bleeding (including history);
- inflammatory bowel diseases: Crohn's disease or ulcerative colitis in the acute phase;
- severe renal failure (creatinine clearance less than 30 ml/min);
- progressive kidney disease;
- severe liver failure;
- complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses and intolerance to ASA or other NSAIDs (including a history);
- established diagnosis of diseases of the blood coagulation system;
- decompensated heart failure;
- therapy of perioperative pain during coronary artery bypass grafting;
- pregnancy;
- breastfeeding period;
- age up to 18 years.
With caution: peptic ulcer of the stomach and duodenum, ulcerative colitis and Crohn's disease without exacerbation, history of liver disease, hepatic porphyria, chronic renal failure (creatinine clearance 30-60 ml/min), chronic heart failure, arterial hypertension, significant decrease in blood volume ( including after surgery), elderly patients (over 65 years old) (including those receiving diuretics, debilitated patients and those with low body weight), bronchial asthma, coronary artery disease, cerebrovascular diseases, dyslipidemia/hyperlipedimia, diabetes mellitus , peripheral arterial diseases, smoking, the presence of Helicobacter pylori infection, long-term use of NSAIDs, alcoholism, severe somatic diseases, autoimmune diseases (systemic lupus erythematosus and mixed connective tissue disease), simultaneous use of corticosteroids (including prednisolone), anticoagulants (in including warfarin), antiplatelet agents (including acetylsalicylic acid, clopidogrel), selective serotonin reuptake inhibitors (including citalopram, fluoxetine, paroxetine, sertraline).
Directions for use and doses
For IM or IV administration.
IM or IV administration is used for short-term (1-2 days) treatment at a dose of 20 mg 1 time/day. If further therapy is necessary, switch to oral dosage forms of tenoxicam.
The injection solution is prepared immediately before use by dissolving the contents of the bottle with the supplied solvent. After preparation, the needle is replaced.
IM injections are made deeply.
The duration of IV administration should not be less than 15 seconds.
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C. Do not freeze.
Best before date
The shelf life of the lyophilisate is 3 years, the solvent is 4 years. Do not use after the expiration date stated on the package.
special instructions
During treatment, it is necessary to monitor the picture of peripheral blood and the functional state of the liver and kidneys, the prothrombin index (while taking indirect anticoagulants), and the concentration of glucose in the blood (while using hypoglycemic agents).
If it is necessary to determine 17-ketosteroids, the drug should be discontinued 48 hours before the study.
Bleeding time may increase, which should be taken into account during surgical interventions.
It is necessary to consider the possibility of sodium and water retention in the body when prescribed with diuretics in patients with arterial hypertension and heart failure.
Patients with uncontrolled arterial hypertension, chronic heart failure, peripheral arterial disease, confirmed coronary artery disease and/or cerebrovascular disease should take the drug under medical supervision.
A history of kidney disease can lead to the development of interstitial nephritis, papillary necrosis and nephrotic syndrome.
Undesirable effects can be minimized by using the minimum effective dose of the drug in the shortest possible course.
Due to the negative effect on fertility, the drug is not recommended for women wishing to become pregnant. In patients with infertility (including those undergoing examination), it is recommended to discontinue the drug.
Patients with systemic lupus erythematosus and mixed connective tissue disease are at increased risk of developing aseptic meningitis.
Description
NSAIDs.
Pharmacodynamics
Tenoxicam is a thienothiazine derivative of oxicam and is an NSAID. In addition to anti-inflammatory, analgesic and antipyretic effects, the drug also prevents platelet aggregation. The mechanism of action is based on inhibition of the activity of the COX-1 and COX-2 isoenzymes, as a result of which the synthesis of prostaglandins in the site of inflammation, as well as in other tissues of the body, is reduced. In addition, tenoxicam reduces the accumulation of leukocytes at the site of inflammation, reduces the activity of proteoglycanase and collagenase in human cartilage.
The anti-inflammatory effect develops by the end of the first week of therapy.
Side effects
Determination of the categories of frequency of side effects (in accordance with WHO recommendations): very often (> 1/10); often (from > 1/100, < 1/10); uncommon (> 1/1000, < 1/100); rare (> 1/10000, <1/1000); very rare (<1/10000), not established.
From the digestive system: very often - dyspepsia (nausea, vomiting, heartburn, diarrhea, flatulence), NSAID gastropathy, abdominal pain, stomatitis, anorexia, liver dysfunction; rarely - ulceration of the gastrointestinal mucosa, bleeding (gastrointestinal, uterine, hemorrhoidal), perforation of the intestinal walls.
From the cardiovascular system: rarely - heart failure, tachycardia, increased blood pressure.
From the side of the central nervous system: often - dizziness, headache, drowsiness, depression, agitation, hearing loss, tinnitus, eye irritation, blurred vision.
From the skin and subcutaneous tissue: often – skin itching, rash, urticaria and erythema; very rarely - photodermatitis, Stevens-Johnson syndrome, Lyell's syndrome.
From the urinary system: often - increased levels of urea nitrogen and creatinine in the blood.
From the hematopoietic organs: often – agranulocytosis, leukopenia; rarely - anemia, thrombocytopenia, leukopenia, pancytopenia.
From the hepatobiliary system: often - increased activity of ALT, AST, GGT and serum bilirubin levels.
From laboratory parameters: hypercreatininemia, hyperbilirubinemia, increased concentration of urea nitrogen and activity of hepatic transaminases, prolongation of bleeding time.
Other: mental disorders and metabolic disorders may occur during treatment.
Use during pregnancy and breastfeeding
The use of the drug during pregnancy and breastfeeding is contraindicated.
Interaction
Tenoxicam is characterized by a high degree of binding to albumin and can, like all NSAIDs, enhance the anticoagulant effect of warfarin and other anticoagulants. It is recommended to monitor blood counts when used together with anticoagulants and hypoglycemic drugs for oral administration, especially in the initial stages of using Artoxan.
No possible interaction with digoxin was noted.
As with other NSAIDs, it is recommended to use the drug with caution concomitantly with cyclosporine due to an increased risk of nephrotoxicity.
Concomitant use with quinolones may increase the risk of seizures.
Salicylates can displace tenoxicam from its binding to albumin and, accordingly, increase the clearance and volume of distribution of the drug. The simultaneous use of salicylates or two or more NSAIDs should be avoided (increased risk of gastrointestinal complications).
There is evidence that NSAIDs reduce lithium excretion. In this regard, in patients receiving lithium therapy, the concentration of lithium in the blood should be monitored more often.
NSAIDs may cause sodium, potassium, and fluid retention in the body, interfering with the action of natriuretic diuretics. This must be remembered when used together with such diuretics in patients with CHF and arterial hypertension.
It is recommended to use NSAIDs with caution in combination with methotrexate; NSAIDs reduce the elimination of methotrexate and may increase its toxicity.
NSAIDs should not be used within 8-12 hours after taking mifepristone, because may reduce its effect.
It is necessary to take into account the increased risk of gastrointestinal bleeding when used together with corticosteroids.
Reduces the effectiveness of uricosuric drugs, enhances the effect of anticoagulants, fibrinolytics, side effects of mineralocorticoids and glucocorticoids, estrogens; reduces the effectiveness of antihypertensive drugs and diuretics.
Inducers of microsomal oxidation in the liver (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants) increase the production of hydroxylated active metabolites.
Combined use with antiplatelet agents and selective serotonin reuptake inhibitors increases the risk of gastrointestinal bleeding.
Cardiac glycosides, when taken together with NSAIDs, may increase heart failure, reduce glomerular filtration rate and increase plasma concentrations of cardiac glycosides.
No interaction has been identified when using tenoxicam with cimetidine.
No clinically significant interactions have been identified during treatment with tenoxicam and penicillamine or parenteral gold preparations.
The risk of nephrotoxicity increases when NSAIDs are used concomitantly with tacrolimus.
The risk of hematological toxicity increases when using NSAIDs with zidovudine.
Overdose
Symptoms (with a single dose): abdominal pain, nausea, vomiting, erosive and ulcerative lesions of the gastrointestinal tract, impaired renal and liver function, metabolic acidosis.
Treatment: symptomatic therapy (maintaining vital functions of the body). Hemodialysis is ineffective.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, the speed of mental and motor reactions may decrease, so it is necessary to refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.