Pharmacological properties of the drug Diferelin
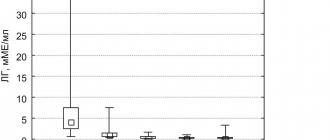
Triptorelin is a synthetic decapeptide, an analogue of the natural gonadotropic RG. After a short initial period of stimulation of the gonadotropic function of the pituitary gland, triptorelin causes inhibition of gonadotropin secretion and, accordingly, the hormone-producing function of the testes or ovaries. With continuous use, the drug inhibits the secretion of estrogen by the ovaries until menopause, and also suppresses the secretion of testosterone, the concentration of which decreases to a level corresponding to the state after surgical castration. Diferelin 0.1 mg After subcutaneous administration of 0.1 mg Diferelin is rapidly absorbed; time to reach the maximum concentration of the drug in the blood plasma - 0.63±0.26 hours; the maximum concentration of the drug in blood plasma is 1.85±0.23 ng/ml. The biological half-life is 7.6±1.6 hours after a 3-4 hour distribution phase. Total plasma clearance - 161±28 ml/min. Conditional volume of distribution is 1562±158 ml/kg. Diferelin 3.75 mg It is a prolonged release drug due to the microspherical structure of triptorelin deposition, which provides a clinically effective concentration for 28 days. After intramuscular administration of a prolonged form of the drug, an initial stage of triptorelin release is observed with further normal release (maximum drug concentration in blood plasma - 0.32 ± 0.12 ng/ml) with an average triptorelin release rate of 46.6 ± 7.1 mg /day Bioavailability: after 1 month - 53%. Diferelin 11.25 mg After intramuscular administration of a dose of Diferelin 11.25 mg, the maximum concentration of triptorelin in the blood plasma is observed after approximately 3 hours. After the phase of decreasing concentration, which occurs during the first month, until the 90th day the level of circulating triptorelin remains constant (approximately 0.03 to 0.06 ng/ml).
Diferelin and beer
Diferelin and beer are incompatible. Triptorelin-based products and alcoholic drinks are prohibited for joint use. It contains hormonal substances that interfere with therapy.
But for this, the patient must be dependent on drinking beer. Alcohol will interfere with the treatment of the underlying disease for which the hormonal drug is used.
It is recommended not to drink alcohol after completing treatment to allow the body time to recover from fluctuations in hormone levels. If the effect decreases, menopause will not occur, causing the disease to progress.
Indications for use of the drug Diferelin
Diferelin 3.75 mg - prostate cancer, genital and extragenital endometriosis (stages I–IV), endometrioid cysts, endometrial hyperplastic processes, female infertility (complex treatment in combination with gonadotropins, to artificially create conditions for ovulation, for the purpose of in vitro fertilization and subsequent embryo transfer ) , premature puberty in children, breast cancer, uterine fibroids. Dipherelin 11.25 mg - adenocarcinoma of the prostate with metastases, when inhibition of testosterone secretion is indicated, premature puberty in children (onset of the disease before 8 years in girls and in boys under 9 years), genital and extragenital endometriosis, breast cancer, when indicated hormonal therapy. Diferelin 0.1 mg - female infertility, ovulation stimulation in combination with gonadotropins for the purpose of in vitro and embryo transplantation (IVFET), as well as in other assisted reproductive technologies.
Diphereline®
Treatment with Diferelin® must be carried out in strict accordance with the instructions for use. Any change in the volume of intramuscularly administered suspension should be recorded.
Diferelin® 3.75 mg contains less than 1 mmol sodium (23 mg) per dose, which is clinically insignificant. Caution should be exercised in patients taking anticoagulants, as hematoma may develop at the injection site.
Endometriosis and treatment of uterine fibroids before surgery
Before starting treatment, pregnancy must be excluded. Regular administration of the drug Diferelin® at a dose of 3.75 mg (one bottle) every four weeks leads to persistent hypogonadotropic amenorrhea. The occurrence of metrorrhagia during treatment, not counting the first month, is not the norm, and therefore it is necessary to determine the concentration of plasma estradiol. If the concentration of estradiol decreases to less than 50 pg/ml, it is necessary to search for other organic lesions.
Given that menstruation should cease during triptorelin therapy, the patient should be instructed to notify her physician if she continues to have regular menstruation.
During treatment, a non-hormonal method of contraception must be used, including 1 month after the last injection.
Ovarian function is restored after cessation of treatment and ovulation occurs approximately 2 months after the last injection. During the treatment of uterine fibroids, it is recommended to regularly determine its size. There are several reports of the development of bleeding in patients with submucosal fibrous fibroids after treatment with GnRH analogues. Bleeding usually developed 6-10 weeks after the start of therapy.
Female infertility
The ovarian response to the administration of triptorelin in combination with gonadotropins may be markedly increased in predisposed patients and in particular in cases of polycystic ovary syndrome. As with the use of other GnRH analogues, cases of ovarian hyperstimulation syndrome associated with the use of triptorelin in combination with gonadotropins have been reported.
The response of the ovaries to the administration of the same doses of triptorelin in combination with gonadotropins may vary among patients, and the reaction may also be different in the same patients during different cycles.
Stimulation of ovulation should be carried out under the supervision of a doctor and with regular laboratory and instrumental examinations: monitoring the content of estrogen in plasma and ultrasound examination. If the ovarian response is excessive, it is recommended to interrupt the stimulation cycle and stop gonadotropin injections.
In patients with renal or hepatic impairment, the average elimination half-life of triptorelin is 7-8 hours, compared with 3-5 hours in healthy individuals. Despite prolonged exposure, triptorelin is not expected to circulate during embryo transfer.
Prostate cancer
The most pronounced beneficial effect of triptorelin therapy is observed in patients in the absence of other previously administered hormonal therapy.
At an early stage, triptorelin, like other GnRH agonists, causes a temporary increase in serum testosterone concentrations. As a result, during the first weeks of therapy, the appearance and intensification of clinical symptoms (in particular, bone pain, dysuric disorders) may be observed, which are transient in nature, and for which symptomatic therapy should be carried out. As with other GnRH agonists, isolated cases of spinal cord compression or urinary tract obstruction may occur. In cases of spinal cord compression or urinary tract obstruction, standard treatment for these complications should be prescribed and, in emergency cases, orchiectomy (surgical castration) should be performed.
Patients should be closely monitored during the first few weeks of therapy (plasma testosterone concentration should not exceed 1 ng/ml), especially patients with metastatic lesions of the spinal cord, patients at risk of spinal cord compression or urinary tract obstruction. For the same reason, special attention should be given at the beginning of treatment to patients with signs of spinal cord compression identified during a preliminary examination. During the initial phase of therapy, the use of additional anti-androgen drugs should be considered to prevent an initial increase in plasma testosterone concentrations and worsening of clinical symptoms.
After surgical castration, triptorelin does not cause a further decrease in plasma testosterone concentrations.
Epidemiological data have shown that patients may develop metabolic abnormalities (such as impaired glucose tolerance) during androgen deprivation therapy; increase the risk of cardiovascular diseases. However, prospective data have not confirmed an association between GnRH agonist treatment and increased cardiovascular mortality. Patients at high risk for metabolic or cardiovascular disease should be carefully assessed before treatment and monitored closely during androgen deprivation therapy.
At the beginning of therapy, a temporary increase in acid phosphatase activity may be observed.
It may be advisable to periodically check the plasma testosterone concentration using an appropriate method, as it should not exceed 1 ng/ml.
Mammary cancer
To ensure adequate suppression in premenopausal women, treatment with triptorelin should be initiated at least 3 to 4 weeks before starting aromatase inhibitor therapy. This is necessary to ensure that circulating estrogen concentrations have reached postmenopausal levels.
Triptorelin should not be interrupted during aromatase inhibitor therapy to avoid a rebound increase in circulating estrogen concentrations in premenopausal patients.
Bone mineral density should be assessed before initiating treatment with triptorelin, especially in women who are at multiple risk of developing osteoporosis. These patients should be closely monitored and begin receiving treatment or prophylaxis for osteoporosis if necessary. Therapy with triptorelin in combination with tamoxifen or aromatase inhibitors in premenopausal women with hormone-dependent breast cancer stages T1-T4, N0-N2a, M0 should be based on a careful individual risk/benefit assessment.
It is recommended that FSH and estradiol concentrations be measured 3–4 weeks after initiation of triptorelin therapy and before initiation of aromatase inhibitor therapy to ensure that circulating estrogen concentrations in the blood are at postmenopausal levels, and such evaluations are recommended to be repeated every three months during the entire period of combination treatment. .
Patients discontinuing triptorelin treatment should also discontinue aromatase inhibitor treatment for 1 month after the last triptorelin injection (for the monthly release form).
The risk of developing musculoskeletal disorders (including stiffness or musculoskeletal pain) when triptorelin is combined with either an aromatase inhibitor or tamoxifen is approximately 89% in combination with an aromatase inhibitor and 76% in combination with tamoxifen).
Hypertension has been reported as an expected adverse event with a very high incidence during triptorelin therapy in combination with either an aromatase inhibitor or tamoxifen. Premenopausal women with breast cancer receiving triptorelin therapy in combination with either an aromatase inhibitor or tamoxifen should have their cardiovascular health and blood pressure monitored regularly.
Hyperglycemia and diabetes have been reported as expected adverse events with high frequency during triptorelin therapy in combination with either an aromatase inhibitor or tamoxifen. Premenopausal women with breast cancer receiving therapy with triptorelin in combination with either an aromatase inhibitor or tamoxifen should be regularly monitored for risk factors for diabetes mellitus by measuring blood glucose on a regular basis and, if necessary, begin appropriate anti-diabetic therapy, in accordance with national guidelines.
Depression occurred in approximately 50% of patients receiving triptorelin in combination with either an aromatase inhibitor or tamoxifen in all treatment arms of the TEXT and SOFT studies, but severe depression (stage 3–4) occurred in less than 5% of patients .
Patients should be informed about the possible development of depression, and if depression develops, should receive appropriate therapy. Patients with previously diagnosed depression should be closely monitored during therapy.
When prescribing triptorelin in combination with an aromatase inhibitor or tamoxifen, special attention should also be paid to the safety information on the use of the aromatase inhibitor and tamoxifen (in accordance with the instructions for the drug that is planned to be used in combination).
Function of the gonads and pituitary gland
The use of triptorelin in therapeutic doses leads to suppression of the hypothalamic-pituitary-ovarian/testicular system. Normal functioning of the gonads and pituitary gland is usually restored after cessation of therapy. The results of a diagnostic test of pituitary gonadotropic function performed during and after cessation of therapy may therefore be incorrect.
Osteoporosis
The use of GnRH agonists may cause a decrease in bone mineral density (BMD).
In men, long-term androgen deprivation with bilateral orchiectomy or GnRH analogues may be associated with an increased risk of bone loss and may lead to osteoporosis and increased risk of bone fracture.
Preliminary data suggest that the use of bisphosphonates in combination with GnRH may reduce BMD loss. Particular attention should be paid to patients with risk factors for the development of osteoporosis (for example: chronic alcohol dependence; smoking; long-term therapy with drugs that reduce BMD, such as anticonvulsants or glucocorticosteroids; a history of hereditary osteoporosis; malnutrition or malnutrition).
In women, the use of GnRH agonists may be responsible for a decrease in BMD by an average of 1% per month (six months of treatment). Every 10% decrease in BMD leads to an increase in the risk of bone fracture by approximately 2-3 times. In most women, current data suggest, BMD recovers after cessation of therapy.
There are no available data on the use of triptorelin in patients with established osteoporosis or with risk factors for osteoporosis (eg, chronic alcohol dependence; smoking; long-term therapy with drugs that lower BMD such as anticonvulsants or glucocorticosteroids; history of hereditary osteoporosis; deficiency or disorder nutrition, for example, anorexia neurosis). Given that a decrease in BMD is most likely in such patients, treatment with triptorelin should be prescribed on an individual basis and should only be initiated if the benefit obtained from treatment outweighs the risk. Additional testing should be considered to avoid loss of BMD.
The use of triptorelin as adjuvant therapy in combination with either tamoxifen or an aromatase inhibitor is associated with a high risk of osteoporosis. Osteoporosis was reported at a higher rate when triptorelin was used in combination with an aromatase inhibitor than with tamoxifen (39% vs. 25%).
Pituitary tumor
Rarely, the use of GnRH agonists may reveal the presence of a previously undiagnosed gonadotropic pituitary adenoma. These patients may develop pituitary hemorrhage, characterized by sudden headache, vomiting, visual disturbances and ophthalmoplegia.
Depression
Patients receiving therapy with GnRH agonists such as triptorelin are at increased risk of developing depression (including severe depression).
Patients should be informed about the possible development of depression, and if depression develops, should receive appropriate therapy. Patients with known depression should be closely monitored during therapy.
QT prolongation
Long-term androgen deprivation may prolong the QT interval. A risk/benefit assessment should be performed before initiating androgen deprivation therapy in patients with congenital long QT syndrome, electrolyte abnormalities, or chronic heart failure, or in patients taking Class IA and III antiarrhythmic drugs.
Precocious puberty
The use of triptorelin in children with precocious puberty should be supervised by a pediatric endocrinologist or pediatrician, or an endocrinologist with experience in the treatment of precocious puberty.
Treatment of children with advanced pituitary tumors should be carried out after an individual assessment of the risk/benefit ratio.
In girls, initial ovarian stimulation, resulting in cessation of estrogen production, may cause mild to moderate vaginal bleeding in the first month, requiring therapy with medroxyprogesterone or cyproterone acetate.
After treatment is completed, pubertal signs develop.
Information on the effect of GnRH analogue therapy on future fertility in patients treated in childhood is still limited. Most girls began regular menstruation an average of one year after the end of therapy.
Precocious puberty due to tumor or hyperplasia of the gonads or adrenal glands, or gonadotropin-independent precocious puberty (testicular damage due to intoxication, hereditary Leydig cell hyperplasia) should be excluded before starting therapy.
BMD may decrease during treatment of precocious puberty with GnRH agonists. However, after cessation of treatment, further bone mass growth is restored, and the therapy does not affect the peak bone mass at the end of puberty.
After completion of therapy with GnRH agonists, epiphysiolysis of the femoral head is possible, because Low concentrations of estrogen during treatment with GnRH agonists weaken the epiphyseal growth plate. An increase in growth rate after the end of therapy leads to displacement of the proximal femoral epiphysis.
Use of the drug Diferelin
Diferelin 3.75 mg The drug is used only intramuscularly. For prostate cancer, Diferelin 3.75 mg of prolonged action is administered intramuscularly every 4 weeks. For endometriosis, endometrioid cysts, endometrial hyperplastic processes, treatment should begin in the first 5 days of the cycle; one IM injection every 4 weeks. The duration of treatment depends on the severity of the disease and the dynamics of clinical manifestations during the treatment period. For the treatment of female infertility, it is prescribed according to the usual therapeutic regimen - one intramuscular injection on the 2nd day of the cycle. Parallel use of gonadotropins begins after achieving pituitary desensitization (the content of estrogens in the blood plasma does not exceed 50 pg/ml), usually on the 15th day after Diferelin injection. It is very important that the injection of the prolonged form is carried out exactly according to the rules specified in the instructions. For premature puberty - one intramuscular injection at a rate of 50 mcg/kg every 4 weeks. For breast cancer - intramuscular injection of Diferelin 3.75 mg of prolonged action every 4 weeks. For uterine fibroids, treatment begins in the first 5 days of the menstrual cycle; Diferelin is administered every 4 weeks. The duration of treatment is 3 months for patients who are being prepared for surgical treatment, and up to 6 months for patients who are not indicated for surgical treatment. Diferelin 11.25 mg For prostate adenocarcinoma - intramuscular injection of Diferelin 11.25 mg prolonged action every 3 months. For endometriosis, treatment begins in the first 5 days of the cycle, one intramuscular injection every 3 months. The duration of treatment depends on the initial severity of endometriosis and the dynamics of clinical manifestations during the period of therapy. The course of treatment is no more than 6 months. For breast cancer - one intramuscular injection every 3 months. For premature puberty - one intramuscular injection every 3 months. Diferelin 0.1 mg 1 ampoule of solvent (1 ml of sodium chloride solution 0.9%) is introduced into a bottle with powder for injection, shake the bottle thoroughly and carefully and immediately make a subcutaneous injection. Short protocol: Diferelin is administered 0.1 mg subcutaneously, starting from the 2nd day of the cycle (simultaneously starting ovarian stimulation) and ending treatment the day before the planned administration of human chorionic gonadotropin. The course of treatment is 10–12 days. Long protocol: injections are carried out subcutaneously daily, starting from the 2nd day of the cycle. After desensitization of the pituitary gland (E2 50 pg/mg, that is, approximately on the 15th day from the start of treatment), ovarian stimulation begins and Diferelin injections are continued at a dose of 0.1 mg/day, ending them the day before the planned administration of human chorionic gonadotropin.
Consequences of administration with alcohol
It is impossible to identify the specific effects of a single dose. Taking into account the increased load on the human body, weakened by pathology, we can talk about an increased risk of side effects.
Due to the specific nature of the pathologies for which this course is prescribed, drinking alcohol contributes to the deterioration of the patient’s well-being. The manufacturer's instructions indicate the likelihood of the condition worsening during the first period of treatment.
If the body at this moment is poisoned by ethanol breakdown products, then the risk of deterioration will increase several times.
Side effects of the drug Diferelin
Manifestations of allergic reactions are possible, such as urticaria, rash, itching, and very rarely - Quincke's edema. Isolated cases of nausea, vomiting, weight gain, increased blood pressure, emotional lability, visual disturbances, pain at the injection site, increased body temperature, and hot flashes have been described. Long-term use of gonadotropin RG analogues can cause bone demineralization and is a possible risk factor for the development of osteoporosis. In men - decreased potency, swelling and tenderness of the mammary glands (noted very rarely). Patients with prostate adenocarcinoma at the beginning of treatment may experience a temporary increase in pain in the bones affected by metastases (treatment is symptomatic). In some cases, ureteral obstruction, as well as symptoms associated with compression of the spinal cord (pass after 1–2 weeks). During this period, a temporary increase in alkaline phosphatase activity in the blood plasma is also possible. In women - sweating and changes in libido, headache, depression, dryness of the vaginal mucosa, changes in the size of the mammary glands. When Diferelin is used in combination with gonadotropins, cases of the development of ovarian hyperstimulation syndrome have been reported. When treating precocious puberty, girls may experience bloody vaginal discharge. Long-term use of the drug may cause hypogonadotropic amenorrhea. After cessation of treatment, ovarian function is restored and ovulation occurs on average on the 58th day after the last injection of the drug.
When can you drink alcohol if you are undergoing or have undergone Deferilin treatment?
This is a course drug; drinking alcohol is allowed only after completion of treatment. The patient should not be embarrassed by cheerful company when undergoing treatment for oncology or infertility. After completing therapy, it makes sense to ask your doctor about the possibility of drinking an alcoholic drink.
The minimum time that should pass depends on the dosage; the maximum presence of the active substance in the body is not indicated by the manufacturer. It is recommended to wait at least a week after stopping treatment.
Alcohol is incompatible with any existing medicine (except for special ones for the treatment of addiction and hangover syndrome).
Special instructions for the use of the drug Diferelin
At the beginning of treatment, clinical symptoms may intensify, and therefore it is necessary to prescribe Diferelin carefully to patients with prostate cancer who have an increased risk of developing ureteral obstruction or spinal cord compression. Such patients should be closely monitored during the first month of treatment. It is necessary to carefully monitor the level of cycle stimulation during in vitro fertilization to identify patients with an increased risk of developing ovarian hyperstimulation syndrome, since the degree and frequency of the syndrome may depend on the gonadotropin dosage regimen. If it is necessary to administer human chorionic gonadotropin, the use of Diferelin should be discontinued. Before starting treatment with Diferelin, the absence of pregnancy should be confirmed.
When can you inject the medicine, after how long
How long after you can inject the medicine depends on the amount of alcohol you drink. Alcohol leaves the human body for a long time.
Body indicators that are important for the time of alcohol withdrawal:
- Body weight.
- Age.
- Liver condition.
- Kidney health.
- Sexual characteristic.
- Associated pathologies.
Therefore, the time for removing alcohol from the body reaches 72-84 hours. During this time, refrain from administering the drug.