Prerequisites for the study
Dapagliflozin is a selective inhibitor of sodium-glucose cotransporter (SGC) type 2, which blocks the reabsorption of glucose in the proximal tubules of the kidneys and promotes glycosuria [1-3]. Previously, data were obtained on the beneficial effect of the use of type 2 NGC inhibitors on the risk of developing complications of cardiovascular diseases (CVD), including a reduction in the risk of hospitalization for heart failure (HF), mainly in patients with diabetes mellitus (DM) 2 type and established diagnosis of CVD [4–6]. In addition, in the course of such studies, data were obtained on slowing the progression of chronic kidney disease (CKD) through the use of type 2 NGC inhibitors [4, 5, 7-9].
Relevance
Patients with chronic kidney disease are at high risk of developing adverse events from the kidneys, blood vessels and heart.
Until now, the effect of dapagliflozin in patients with chronic kidney disease with or without diabetes mellitus was not known.
Sick
The study included patients with type 2 diabetes aged 40 years whose characteristics met the following criteria:
- level of glycated hemoglobin (HbA1c) 6.5% or more, but less than 12%;
— creatinine clearance 60 ml/min or more;
— several risk factors (RFs) for the development of CVD or an established diagnosis of CVD, cerebrovascular disease (clinically obvious coronary heart disease, cerebrovascular disease of ischemic nature or peripheral artery disease);
- in the presence of only risk factors for the development of CVD, the age of men and women should have been at least 55 and 60 years, respectively, if they had at least 1 known risk factor, including arterial hypertension, dyslipidemia (low-density lipoprotein cholesterol more than 3.36 mmol/l or use of lipid-lowering therapy) and tobacco use.
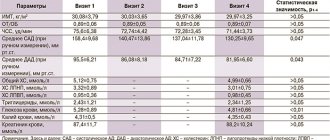
The baseline characteristics of the study participants are presented in detail in the table.
Table. Baseline characteristics of study participants Note. Data are presented as mean ± standard deviation or * - median (minimum value - maximum value) unless otherwise noted. DM - diabetes mellitus; BP—arterial hypertension; GFR—glomerular filtration rate; BSA—body surface area; CVD is a cardiovascular disease caused by atherosclerosis; CHD - coronary heart disease; PAD—peripheral artery disease; CVD - cerebrovascular diseases; DPP-4 - dipeptidyl peptidase type 4 inhibitors; GLP-1 - glucagon-like peptide type 1; CVD - cardiovascular disease; ACE - angiotensin-converting enzyme; ARBs—angiotensin II receptor blockers.
Material and methods
A single-center prospective study was conducted, approved by the ethics committee of the Federal State Budgetary Institution “National Medical Research Center named after. V.A. Almazov" of the Russian Ministry of Health. Before entering the study, all patients signed informed consent. Patients were included with T2DM lasting more than 1 year, glycated hemoglobin level from 7% to 10%, without verified atherosclerotic heart and vascular disease, but with multiple risk factors for cardiovascular events (dyslipidemia, obesity, arterial hypertension). In addition, inclusion criteria were age from 40 to 65 years, stable hypoglycemic, lipid-lowering and antihypertensive therapy for at least 12 weeks. before inclusion in the study. The study did not include patients with atrial fibrillation, valvular heart disease, rheumatological diseases, exacerbation of chronic diseases, type 1 diabetes mellitus, symptoms of hypotension, and systolic blood pressure (SBP) levels below 95 mmHg. Art., the level of N-terminal propeptide of natriuretic hormone (NT-proBNP) >125 ng/ml, glomerular filtration rate (GFR) <60 ml/min/1.73 m2 and chronic kidney disease, liver disease and also receiving insulin therapy, therapy glucocorticosteroids, mineralocorticoid receptor antagonists. Patients received dapagliflozin at a dose of 10 mg/day for 6 months. in addition to basic therapy for T2DM.
During the study, blood samples were collected before starting treatment with dapagliflozin and after 6 months. treatment. Blood samples were taken on an empty stomach (after 8 hours of fasting), and the following parameters were assessed: glycated hemoglobin (HbA1c), alanine aminotransferase, aspartate aminotransferase, creatinine, lipid profile (total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides), NT -proBNP, hsCRP, galectin-3, tissue inhibitor of matrix metalloproteinases-1 (TIMP-1), matrix metalloproteinase-9 (MMP-9), stimulating growth factor expressed by gene 2 (ST2), carboxy-terminal fragment of type I procollagen (PIСP) . The hsCRP level was determined on a Cobas Integra 400+ analyzer using the immunoturbidimetric method. The concentration of NT-proBNP was determined by the electrochemiluminescent method using the Elecsys test system (Roche Diagnostic). Serum levels of galectin-3 (R&D system), MMP-9 and TIMP-1 (R&D system), sST2 (Clinical diagnostics, Presage ST2 kit), PICP (USCN Life Science) were assessed by enzyme immunoassay. In addition, body weight, height, waist circumference, and blood pressure (BP) were assessed at the beginning and end of the study. In order to assess the normal values of fibrosis biomarkers, the study additionally included 15 patients of a similar age without T2DM and other chronic diseases, including cardiovascular and kidney diseases (control group).
The SPSS Statistics program was used for statistical analysis. Quantitative data are presented as median and 25th and 75th quartiles - Me [Q25; Q75], qualitative characteristics - in the form of absolute and relative (%) indicators. Differences between quantitative indicators were identified using the Wilcoxon test. Correlation analysis was performed using Spearman's rank correlation coefficient. The null hypothesis was rejected at p<0.05.
Intervention
During the run-in period, all participants received a placebo in a single-blind manner and had blood and urine tests performed. After this period, participants who continued to meet the inclusion and exclusion criteria were randomized 1:1 in a double-blind manner to receive dapagliflozin 10 mg once daily or placebo. Other hypoglycemic drugs (except for the unblinded type 2 NGC inhibitors, pioglitazone or rosiglitazone) were used at the discretion of the treating physician. Participants were required to visit the study site every 6 months until study completion and laboratory and clinical assessments of safety and adherence were obtained. In addition, participants were contacted by telephone every 3 months between study site visits.
Evaluation criteria/Clinical outcomes
The main combined safety indicator: the incidence of severe complications of cardiovascular diseases - ASCVD (death from CVD complications, myocardial infarction - MI or ischemic stroke).
Key combined performance indicators:
— incidence of ASCVD (death from complications of CVD, MI or ischemic stroke);
— mortality from complications of CVD and the frequency of hospitalizations for HF.
Additional performance indicators:
- a combined indicator of the incidence of CKD (a decrease of 40% or more in the estimated glomerular filtration rate to less than 60 ml/min per 1.73 m2, newly developed end-stage renal failure, or death from CKD or CVD);
- total mortality.
Statistical analysis methods
Safety was assessed using an analysis designed to test the hypothesis that dapagliflozin was no less safe than placebo in terms of its effect on the risk of ASCVD. In accordance with the recommendations of the US Food and Drug Administration, the results of such an analysis should indicate that the upper limit of the 95% CI of the risk ratio for ASCVD for a two-sided criterion is 1.3 with a one-sided level of α = 0.023 (after accounting for the results 2 interim analyses). If the hypothesis of non-inferior safety of dapagliflozin use compared to placebo was confirmed, it was planned to evaluate 2 effectiveness indicators: the incidence of ASCVD and a combined indicator of the incidence of such outcomes as death from CVD complications and hospitalization for CVD. The assessment of these indicators was required to be parallel at a two-sided level of α = 0.023 for each. If there was a statistically significant decrease in any of these measures, the α level could be adjusted to test other performance measures at α = 0.046 for a two-tailed test. In case of statistically significant effectiveness on both indicators, additional indicators were analyzed using a hierarchical approach at the α=0.046 level for both indicators.
According to the original study protocol, approximately 17,150 participants were required to be included in the study with a follow-up of at least 3 years for at least 1,390 adverse clinical outcomes to occur. This number of participants provided 85% statistical power to detect a 15% reduction in the incidence of ASCVD in the dapagliflozin group compared with the placebo group at α = 0.046 for a two-sided test, and more than 99% statistical power to support the hypothesis of non-inferiority of safety use of dapagliflozin compared with placebo, which was assessed for the effect on the risk of developing ASCVD.
The analysis of key safety and efficacy outcomes included data from 17,160 participants who were randomized. Data from 30 participants enrolled in the single-center study were excluded from the analysis. The reason for excluding these data was that there were significant violations of the principles of Good Clinical Practice in the specified center during the implementation of another study, which was the basis for doubts about the reliability of the data obtained.
All analyzes were performed on the assumption that all study participants were assigned to treatment, using validated clinical outcome data. For the main outcome measures, hazard ratios and 95% CIs were provided with p values obtained from analyzes of duration to adverse clinical outcomes. This analysis was performed using a Cox proportional hazards model, which included data from all participants. All analyzes were performed using stratification based on the presence or absence at study entry of CVD (established diagnosis of CVD or multiple risk factors for CVD) and hematuria. After completing the safety analysis (incidence of ASCVD), other safety outcomes were assessed, which included data from the participants assessed for safety (all participants who received at least one dose of dapagliflozin or placebo). For safety indicators, the p value calculated without taking into account multiple comparisons was reported.
Discussion
The predominance of type I collagen synthesis over its degradation leads to the accumulation of excess collagen fibers in the myocardium and indicates the process of fibrosis that occurs in the interstitial and perivascular space, including in diabetic cardiomyopathy [9]. Levels of PICP, which is a marker of type I collagen formation and degradation, reliably correlate with the amount of collagen formed in both patients with and without chronic heart failure (CHF) [9].
Studies have shown that PICP concentrations are higher in patients with T2DM than in patients without T2DM [10]. Similar differences were demonstrated in our study. Therapy with dapagliflozin for 6 months. led to a significant decrease in P1CP levels compared to baseline. Similar dynamics were obtained in a study of another INGT2 in patients with T2DM and a very high risk of cardiovascular events [11].
Our study did not establish a significant decrease in NT-proBNP concentrations during treatment with dapagliflozin for 6 months, which can be explained by the population of patients included in the study without CHF and without high NT-proBNP concentrations. Since NT-proBNP is a marker of CHF and its level correlates with its severity, even in studies of patients with T2DM and CHF with preserved ejection fraction, no significant decrease in NT-proBNP concentration was detected [12]. However, the DAPA-HF trial demonstrated that the risk of worsening CHF or cardiovascular death was lower in the dapagliflozin group than in the placebo group [13]. Thus, despite having a limited effect on NT-proBNP levels, INGT2 improves clinical outcomes in patients with CHF, suggesting a discrepancy between short-term changes in NT-proBNP levels and clinical outcomes.
The concentrations of ST2, hsCRP, MMP-9 and its inhibitor TIMP-1 did not change significantly during dapagliflozin therapy, which is consistent with data from other studies [11, 14, 15]. Probably, the lack of significant dynamics in the indicators of a number of markers during our study is due to the fact that patients without CHF and cardiovascular events are at that stage of the cardiovascular continuum when the concentrations of markers cannot fully reflect the severity and prognosis of these patients. and their changes cannot be recorded over a 6-month observation period. In addition, the absence of negative dynamics in the content of these biomarkers may indicate a slowdown in the inflammatory and fibrotic processes that occur in T2DM and potentially translate into improved cardiovascular outcomes. It is also worth taking into account the presence of obesity and other unaccounted factors that may influence the values of the markers we evaluate.
Main results
A total of 25,698 participants were included in the placebo run-in phase of the study. After completion of this period, randomization was performed and 17,160 participants were enrolled in the main part of the study using a double-blind design (10,186 participants did not have a diagnosed ASCVD at entry). The dapagliflozin group and placebo group included 8582 and 8578 participants, respectively.
Data from the primary safety outcome analysis indicated that dapagliflozin met pre-specified analysis requirements to test the hypothesis that dapagliflozin is noninferior to placebo as measured by the risk of ASCVD (upper limit 95). The % CI of the risk ratio was required to be less than 1.3 at p < 0.001 for the analysis performed to test the hypothesis that dapagliflozin was noninferior to safety compared with placebo).
The analysis of two main efficacy endpoints showed that dapagliflozin did not reduce the incidence of ASCVD compared with placebo (8.8% and 9.4% of participants in the dapagliflozin group and placebo group, respectively; hazard ratio: 0. 93 with 95% CI from 0.84 to 1.03; p = 0.17), but caused a decrease in the combined mortality rate from CVD complications and the frequency of hospitalizations for HF (such outcomes in the dapagliflozin group and the placebo group developed in 4.9 and 5.8% of participants, respectively; hazard ratio 0.83, 95% CI 0.73 to 0.95; p=0.005), reflecting lower rates of hospitalization for HF (hazard ratio 0.73, 95% CI from 0.61 to 0.88) in the absence of statistically significant differences between groups in mortality from CVD complications (hazard ratio 0.98 with 95% CI from 0.82 to 1.17). Renal failure in the dapagliflozin group and placebo group occurred in 4.3 and 5.6% of participants, respectively (hazard ratio 0.76, 95% CI 0.67 to 0.87), and overall mortality was 6.2 and 6. 6%, respectively (hazard ratio 0.93, 95% CI 0.82 to 1.04). The incidence of diabetic ketoacidosis was higher in the dapagliflozin group compared with the placebo group and was 0.3% and 0.1%, respectively (p=0.02), as was the incidence of genital infection that led to discontinuation of study drug or was considered severe adverse event - 0.9 and 0.1%, respectively (p<0.001).
Research results
The study included 27 patients (age 56 [49; 61] years), most of whom had T2DM lasting more than 4 years (7 [4; 12] years) and stage I obesity. Basic hypoglycemic therapy included a combination of metformin with sulfonylureas or dipeptidyl peptidase type 4 inhibitors (DPP-4 inhibitors).
The main characteristics of the patients and the dynamics of the studied markers of fibrosis and inflammation are presented in Table 1. Over 6 months. treatment with dapagliflozin there was a significant decrease in HbA1c levels from 8.2 [7.6; 8.8]% to 7.8 [7.2; 8.1]% (p=0.001), decrease in BMI and decrease in waist circumference. During the observation period, there were no significant changes in the content of lipoproteins and triglycerides, GFR and blood pressure.
The content of hsCRP and NT-proBNP did not differ from normal values and did not undergo statistically significant changes after 6 months. treatment with dapagliflozin. At baseline, patients had significantly higher concentrations of galectin-3, PICP and MMP-9 compared to the control group (p=0.017, p=0.006 and p=0.008, respectively). When assessing the dynamics of the content of galectin-3, MMP-9, TIMP-1, ST2, no significant differences with the initial values were obtained (see Table 1). At the same time, after 6 months. treatment with dapagliflozin there was a statistically significant decrease in PICP concentration from 136.8 [100.4; 200.6] ng/ml to 104.8 [79.7; 162.0] ng/ml (p=0.019) and an increase in TIMP-1 concentration from 188 [138; 270] ng/ml up to 234 [205; 315] ng/ml (p=0.011).