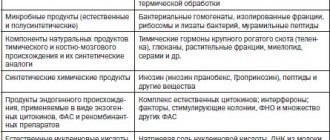
Isoprinosine
Immunostimulating agent with nonspecific antiviral effect. It is a complex containing inosine and a salt of 4-acetamidobenzoic acid with N,N-dimethylamino-2-propanol in a molar ratio of 1:3. The effectiveness of the complex is determined by the presence of inosine; the second component increases its availability to lymphocytes. Restores the functions of lymphocytes in conditions of immunosuppression, increases blastogenesis in the population of monocytic cells, stimulates the expression of membrane receptors on the surface of T-helper cells, prevents a decrease in the activity of lymphocyte cells under the influence of glucocorticoids, and normalizes the inclusion of thymidine in them. Isoprinosine has a stimulating effect on the activity of cytotoxic T-lymphocytes and natural killer cells, the functions of T-suppressors and T-helpers, increases the production of IgG, interferon gamma, interleukins (IL)-1 and IL-2, reduces the formation of pro-inflammatory cytokines - IL-4 and IL-10 potentiates the chemotaxis of neutrophils, monocytes and macrophages.
Shows antiviral activity in vivo against Herpes simplex viruses, cytomegalovirus and measles virus, human T-cell lymphoma virus type III, polioviruses, influenza A and B, ECHO virus (human enterocytopathogenic virus), encephalomyocarditis and equine encephalitis. The mechanism of antiviral action is associated with inhibition of viral RNA and the enzyme dihydropteroate synthetase, which is involved in the replication of some viruses. Enhances the synthesis of lymphocyte mRNA suppressed by viruses, which is accompanied by suppression of the biosynthesis of viral RNA and translation of viral proteins, increases the production of alpha and gamma interferons by lymphocytes, which have antiviral properties
Reduces the clinical manifestations of viral diseases, accelerates convalescence, and increases the body's resistance.
When used as an auxiliary drug for infectious lesions of the mucous membranes and skin caused by the Herpes simplex virus, the affected surface heals more quickly than with traditional treatment. New blisters, swelling, erosions and relapses of the disease occur less frequently. With timely use of inosine pranobex, the incidence of viral infections decreases, the duration and severity of the disease decreases.
Pharmacokinetics
After oral administration, it is quickly and almost completely (> 90%) absorbed and has good bioavailability. When taken orally at a dose of 1500 mg, the Cmax of inosine pranobex is achieved after 1 hour and is 600 mcg/ml. Not detectable in the blood 2 hours after administration.
Inosine pranobex consists of inosine and the salt of p-acetamidobenzoic acid with N,N-dimethylamino-2-propanol. Each of the components of inosine pranobex is rapidly metabolized. Almost 100% of metabolites are detected in urine within 8 to 24 hours after administration. Inosine undergoes metabolism in a cycle typical of purine nucleotides, producing uric acid, the concentration of which in the blood serum may increase. As a result, uric acid crystals may form in the urinary tract. The increase in uric acid concentration is nonlinear and can vary by ±10% within 1-3 hours after ingestion. As a result of the metabolism of p-acetamidobenzoic acid, o-acyl glucuronide is formed; N,N-dimethylamino-2-propanol is metabolized to N-oxide. AUC of p-acetamidobenzoic acid >88%, AUC of N,N - dimethylamino-2-propanol >77%. No accumulation was detected in the body.
Inosine and its metabolites are excreted in the urine. When equilibrium concentration is reached when taking a daily dose of 4 g, the daily urinary excretion of p-acetamidobenzoic acid and its metabolite is approximately 85% of the dose taken; T1/2 - 50 min. T1/2 N,N-dimethylamino-2-propanol - 3-5 hours.
Inosine pranobex and its metabolites are completely eliminated from the body within 48 hours.
Indications
- Viral infections caused by herpes simplex virus types 1 and 2, varicella zoster virus, cytomegalovirus, Epstein-Barr virus, measles virus, mumps virus, including in patients with immunodeficiency conditions; viral respiratory infections; papillomavirus infections of the skin and mucous membrane: genital warts, papillomavirus infection of the vulva, vagina and cervix (as part of complex therapy); acute viral encephalitis (as part of complex therapy); viral hepatitis (as part of complex therapy); subacute sclerosing panencephalitis (as part of complex therapy).
Note!
Description of the drug Isoprinosine table. 500 mg No. 50 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Interactions
The drug should not be used simultaneously with immunosuppressants. the drug is prescribed with caution with xanthine oxidase inhibitors or agents that promote the excretion of uric acid, including diuretics, in particular with thiazide diuretics (such as hydrochlorothiazide, chlorthalidone, indapamide) or loop diuretics (for example, furosemide, torsemide, ethacrynic acid).
When used concomitantly with zidovudine (azidothymidine), the formation of zidovudine nucleotide is increased through many mechanisms, in particular due to an increase in the bioavailability of azidothymidine in blood plasma and intracellular phosphorylation in human blood monocytes. The result of this is an increase in the effect of zidovudine.
special instructions
During treatment with isoprinosine, a temporary increase in the level of uric acid in the blood plasma and urine is possible, especially in men and the elderly, however, these indicators usually remain within normal limits (up to 8 mg/ml in the blood plasma).
The cause of increased uric acid levels is the catabolic metabolism of inosine in humans. This is not due to a drug-induced fundamental change in enzyme or renal clearance function. Therefore, the drug should be used with extreme caution in patients with gout, hyperuricemia, urolithiasis and reduced renal function. During treatment, it is necessary to monitor uric acid levels in these patients. Some patients may experience acute hypersensitivity reactions (angioedema, anaphylactic shock, urticaria). In this case, Isoprinosine therapy should be discontinued.
With long-term use of the drug, there is a risk of developing nephrolithiasis.
Isoprinosine contains wheat starch, which may contain gluten, but in extremely small quantities, so the drug is considered safe for people with celiac disease (celiac disease). Patients with a wheat allergy (as opposed to celiac disease) are not recommended to take this drug.
Isoprinosine contains mannitol, which can have a laxative effect.
Use during pregnancy and lactation. Research into the possible risk of pathologies in the fetus and fertility disorders in humans has not been conducted. It is not known whether inosine pranobex passes into breast milk. It is not recommended to use the drug during pregnancy and breastfeeding.
Children. The drug is used in children over 1 year of age.
The ability to influence reaction speed when driving vehicles or working with other mechanisms. The effect of the drug on reaction speed when driving vehicles or working with other mechanisms has not been studied. However, patients should be aware that the drug may cause dizziness or other adverse reactions from the nervous system.
Application
The drug is prescribed orally. The daily dose depends on body weight, the course and severity of the disease, and the patient’s condition.
Adults and children over 12 years of age: 50 mg/kg body weight (6-8 tablets, divided into 3-4 doses), maximum daily dose - 4 g.
Children aged 1 to 12 years: 50 mg/kg body weight (1 tablet per 10 kg body weight for a child weighing 10–20 kg, for a body weight of 20 kg, prescribe the dose as for adults), divided into 3 –4 doses per day, maximum daily dose - 4 g. To make swallowing easier, the tablet can be crushed.
Duration of treatment
Acute diseases: for diseases with a short-term course, the course of treatment is from 5 to 14 days. After the severity of the symptoms of the disease has decreased, treatment should be continued for another 1–2 days or longer, depending on the course of the disease and the patient’s condition.
Viral diseases with a long course: treatment should be continued for 1-2 weeks after the severity of the symptoms of the disease has subsided or longer depending on the course of the disease and the patient’s condition.
Recurrent diseases: at the initial stage of treatment, the same recommendations apply as for acute diseases. During maintenance therapy, the dose can be reduced to 500–1000 mg (1–2 tablets) per day. When the first signs of relapse appear, it is necessary to resume taking the daily dose recommended for acute diseases and continue use for 1-2 days after the symptoms disappear. The course of treatment can be repeated several times if necessary and depending on the patient’s condition on the recommendation of a doctor.
Chronic diseases: use the drug in a daily dose of 50 mg/kg body weight according to the following schemes:
- asymptomatic diseases - take for 30 days with a break of 60 days;
- diseases with moderately severe symptoms - take for 60 days with a break of 30 days;
- diseases with severe symptoms - apply for 90 days with a break of 30 days.
If necessary, the course of treatment should be repeated under the supervision of a physician, and it is necessary to constantly monitor the patient’s condition and the course of the disease to continue therapy.
For infections caused by the human papillomavirus (external genital warts (genital warts) or human papillomavirus infection of the cervical canal), take 3 g (2 tablets 3 times a day) for 14–28 days as monotherapy or as an addition to local therapy or surgical treatment according to the following schemes:
a) for the treatment of low-risk patients (patients with normal immunity or patients with a low risk of relapse), the drug is used for 14–28 days until maximum eradication of the virus is achieved, then a break should be taken for 2 months. The course of treatment can be repeated using the same dose, but constant monitoring of the patient’s condition and the course of the disease is necessary to prolong therapy;
b) for the treatment of high-risk patients* (patients with immunodeficiency or at high risk of relapse), the drug is used 5 days a week, consistently 1-2 weeks a month for 3 months. If necessary, the course of treatment should be repeated, and constant monitoring of the patient's condition and the course of the disease is necessary to prolong therapy.
*High-risk factors in patients with recurrent or cervical dysplasia or genital human papillomavirus infection, as with other similar diseases, include:
- immunodeficiency caused by a history of chronic or recurrent infections or sexually transmitted diseases; chemotherapy; chronic alcoholism;
- long-term use of oral contraceptives (≥2 years);
- folate level in erythrocytes 660 nmol/l;
- several sexual partners or a change of permanent sexual partner;
- frequent vaginal intercourse (≥2–6 times per week) or anal sex;
- atopy (hereditary predisposition to hypersensitivity);
- poorly controlled diabetes;
- smoking;
- papillomavirus infection of the genitals, which lasts 2 years or has ≥3 relapses in history;
- negative history of skin warts in childhood.
For subacute sclerosing panencephalitis, the daily dose is 100 mg/kg body weight, the maximum dose is 3–4 g/day, and constant monitoring of the patient’s condition and indications for prolongation of therapy are necessary.
Side effects
The only side effect that most often occurs during treatment with isoprinosine in both adults and children is a temporary increase in uric acid levels in the blood plasma and urine (usually the level is within the normal range and returns to baseline values a few days after the end of treatment).
From the immune system: angioedema, hypersensitivity, urticaria, anaphylactic reactions.
Mental disorders: nervousness.
From the nervous system: headache, vertigo, drowsiness, insomnia, dizziness.
From the gastrointestinal tract: nausea, vomiting, discomfort in the epigastric region, diarrhea, constipation, abdominal pain (in the upper abdomen).
From the skin and subcutaneous tissue: rash, itching, erythema.
From the musculoskeletal system and connective tissue: arthralgia.
From the kidneys and urinary system: polyuria.
General disorders and conditions: fatigue, malaise.
Laboratory data: increased levels of uric acid in the blood and urine; increased levels of urea, transaminases, alkaline phosphatase in the blood.