Aluminum phosphate
(aluminum orthophosphate, aluminum phosphate) - AlPO4, inorganic compound, aluminum salt of phosphoric acid. A solid, white crystalline substance, insoluble in water. Occurs in nature in the form of numerous minerals. It is formed in the form of a gelatinous precipitate when water-soluble aluminum salts are exposed to soluble phosphates.
It is used as a flux in the production of ceramics, an additive for cement, a high-temperature dehydrating agent, for the production of special types of glass, as a catalyst in organic synthesis. It is also used as a component for some baking powders in confectionery and in medicine as an antacid.
Chemical properties
Aluminum orthophosphate is an inorganic compound, a white, solid that is insoluble in water and poorly soluble in alcohol. The product dissolves well in nitric and hydrochloric acid . Aluminum Phosphate Formula: AlPO4. The chemical compound is found in various natural minerals. The substance is a reaction product of the action of water-soluble aluminum salts on soluble phosphates. This produces a white gelatinous precipitate.
There are 4 modifications of the Aluminum Phosphate formula, among which the most stable are: α-AlPO4 - with a hexagonal lattice and β-AlPO4 - with a hexagonal or cubic lattice. The chemical compound is quite stable and decomposes at very high temperatures (above 2000 degrees). Molecular weight = 121.9 grams per mole.
The substance is used as a flux in the production of ceramic products; added to cement during construction; used as a high-temperature dehydrating agent in the glass industry; as a catalyst during organic synthesis. Aluminum phosphate is used as a leavening agent in the manufacture of confectionery products. In medicine, the drug is prescribed as an antacid.
use
Aluminum orthophosphate is used as a flux in the production of glass, ceramics and glazes. When mixed with calcium sulfate and sodium silicates, it is known as cement
.
Gels containing aluminum orthophosphate are used in medicine as antacids.
It is also used as an adjuvant in some vaccines, such as the pneumococcal Synflorix vaccine
or
Prevenar 13,
or as a combination of AS04 in hepatitis B and HPV vaccines. It is in a chemically amorphous form in which some OH groups are replaced by phosphate groups. This results in a large surface area and absorption capacity. The isoelectric point of commercially available adjuvant ranges from 4.5 to 5.5.
Pharmacodynamics and pharmacokinetics
After entering the stomach, Aluminum Phosphate within 10 minutes increases the pH level to 3.5-5 and significantly reduces the activity of the pepsin . The substance does not cause alkalization of gastric juice, the acidity of the stomach contents remains at the same level, and does not stimulate secondary hypersecretion of hydrochloric acid . The medicine has an absorbent effect, eliminating viruses, bacteria, exotoxins , gases and endotoxins . After taking this medicine, a protective layer forms on the mucous membrane of the digestive tract.
Aluminum phosphate
Aluminum phosphate
(eng.
aluminum phosphate
) or
aluminum orthophosphate
,
aluminum phosphate , AlPO4
- aluminum salt of phosphoric acid. In medicine, it is an antacid.
Aluminum phosphate is the international nonproprietary name of the drug
Aluminum phosphate is the international nonproprietary name (INN) of the drug. According to ATC, aluminum phosphate belongs to the section “A02A Antacids”, group “A02AB Aluminum Preparations” and has the code A02AB03.
Aluminum phosphate - antacid
Aluminum phosphate, as an antacid, is a so-called “non-absorbable antacid”.
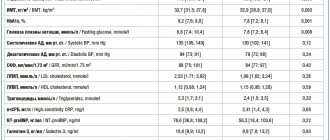
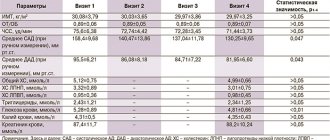
The effect of non-absorbable antacids develops more slowly than that of absorbed ones, but lasts longer, up to 2.5–3 hours. Another important advantage of non-absorbable antacids is the absence of the “acid rebound” phenomenon, which consists of an increase in the secretion of hydrochloric acid after the end of the drug’s effect (Bordin D.S.). Most of the aluminum phosphate gel is insoluble, but at less than 2.5 the aluminum phosphate gel turns into water-soluble ammonium chloride, part of which is able to dissolve, after which further dissolution of the aluminum phosphate is suspended. A gradual decrease in the acidity level of gastric contents to pH 3.0 does not lead to the occurrence of “acid rebound”: the use of aluminum phosphate gel does not lead to the appearance of secondary hypersecretion of hydrochloric acid. Colloidal aluminum phosphate binds endogenous and exogenous toxins, bacteria, viruses, gases formed as a result of putrefaction and pathological fermentation throughout the gastrointestinal tract, normalizing their passage through the intestines and thereby facilitating their removal from the body. Under its influence, pain sensations are also weakened (Vasiliev Yu.V.). Aluminum phosphate in a dose of 2.08 g is used in the treatment of moderate and infrequently occurring symptoms of gastroesophageal reflux disease (GERD), especially those associated with non-compliance with the recommended lifestyle (Recommendations of the Russian State Administration for the diagnosis and treatment of GERD, 2020).
The advantage of aluminum phosphate compared to other non-absorbable antacids containing aluminum
The level of aluminum absorption may be different for different drugs, which must be taken into account when determining the possible risk of side effects due to the fact that antacid drugs containing aluminum can cause hypophosphatemia in some patients, especially with prolonged use; in renal failure - encephalopathy, osteomalacia (with an aluminum level of more than 3.7 µmol/l), clinical symptoms considered characteristic of poisoning (with an aluminum concentration of more than 7.4 µmol/l).
The lower toxicity of aluminum phosphate, compared to aluminum hydroxide, is due to its greater resistance to dissolution and the formation of neutral complexes in the presence of acids usually found in food, which indicates the lower toxicity of aluminum phosphate (Vasiliev Yu.V.). With long-term use of drugs based on aluminum hydroxide, which interferes with the absorption of dietary phosphates in the intestine, hypophosphatemia, osteoporosis and osteomalacia may develop. This circumstance is associated with the limitation of the use of aluminum-containing antacids in children and pregnant women. The exception is aluminum phosphate, which does not affect phosphorus-calcium metabolism. Therefore, it can be prescribed to both pregnant women and nursing mothers, and children from birth. The use of aluminum phosphate may be preferable in elderly patients, who often have reduced bone density (Samsonov A.A., Odintsova A.N.).
Professional medical articles addressing the use of aluminum phosphate
- Vasiliev Yu.V. Modern antacid drugs in gastroenterological practice // Attending Physician. – 2004. – No. 4.
- Konorev M.R. Selection of the optimal antacid drug in clinical practice // Consilium Medicum. – 2003. Extra edition. – pp. 9–11.
- Lapina T.L. The importance of antacids in acid-dependent diseases // Breast Cancer. Diseases of the digestive system. – 2006. – Volume 8. – No. 2. – p. 114–116
On the website in the literature catalog there is a section “Antacids”, containing articles devoted to the treatment of diseases of the gastrointestinal tract with antacids
Medicines with the active ingredient aluminum phosphate
Currently, only one medicine with aluminum phosphate as the only active ingredient is registered in Russia - Phosphalugel.
Previously, Alfogel and Gasterin (also containing pectin) also had registration, but this has now expired. Hephal, a drug with the active ingredient aluminum phosphate, is produced in Belarus.
In some countries, medicinal products containing the following active ingredients are (and were previously approved for) sale:
- aluminum phosphate: Alfogel, Gelfos (Gelfos), Gelatum, Phosphalugel
- aluminum phosphate + simethicone: Alposim, Aluphagel
- aluminum phosphate + pectin: Gasterin.
Aluminum phosphate has contraindications, side effects and application features; consultation with a specialist is necessary. Some manufacturers do not recommend that pregnant women use drugs containing aluminum phosphate, as stated in the instructions (Tyutyunnik V.L., Elokhina T.B.). Back to section
Indications for use
Gel with Aluminum Phosphate is prescribed:
- for the treatment of gastric and duodenal ulcers during exacerbation;
- for chronic gastritis with increased or normal secretory function in the acute phase;
- patients with acute duodenitis , gastritis ;
- with erosion of the gastrointestinal mucosa;
- for the treatment of reflux esophagitis , hiatal hernia ;
- for enterocolitis , proctitis , sigmoiditis , diverticulitis ;
- to eliminate diarrhea after gastrectomy ;
- patients with indigestion , including after taking medications, chemotherapy , non-compliance with diet, neurotic origin;
- in acute or chronic pancreatitis (exacerbation phase);
- patients with intoxication and poisoning.
Links[edit]
- Dec, Corbridge. (2013). Phosphorus: chemistry, biochemistry and technology
(6th ed.). CRC Press. ISBN 9781439840894.
Quotes [edit]
- Patnaik will die. Handbook of Inorganic Chemicals
. McGraw-Hill, 2002, ISBN 0-07-049439-8 - ^ a b Corbridge, pp. 207-208
- Corbridge, page 310
- Tanaka, Y; and others. (2010). "Determination of structural chirality of berlinite and quartz by resonant x-ray diffraction with circularly polarized x-rays". Physical Review
B.
81
(14): 144104. Bibcode: 2010PhRvB..81n4104T. DOI: 10.1103/PhysRevB.81.144104. ISSN 1098-0121. - Growing crystals from a piezoelectric α-quartz-like material, berlinite, Motchany AI, Chvanski PP, Annales de Chimie Science des Materiaux properties, 2001, 26, 199
- ^ab Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of elements
(2nd ed.). Butterworth-Heinemann. p. 527. ISBN 978-0-08-037941-8. - Wilson, S.T.; and others. (1982). "Aluminophosphate molecular sieves: a new class of microporous crystalline inorganic solids". Journal of the American Chemical Society
.
104
(4):1146–1147. DOI: 10.1021/ja00368a062. ISSN 0002-7863. - Kulprathipanja, S, ed. (2010-02-17). Zeolites in industrial separations and catalysis
. John Wiley and Sons. DOI: 10.1002/9783527629565. ISBN 9783527325054. - Xu, R; and others. (2007). Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure
. John Wiley and Sons. paragraph 39. ISBN 9780470822333. - Jump up
↑ RJ, Crowther (2010).
Vaccine adjuvants: preparation methods and research protocols
. Humana. pp. 65–66, 82. ISBN 9781617371592. - ^ a b Schaefer, Christophe; Peters, Paul W.J.; Miller, Richard K. (2015). Medications in Pregnancy and Breastfeeding: Treatment Options and Risk Assessment
. S. Schaefer, P. Peters, R. C. Miller (3rd ed.). paragraph 94. ISBN 9780124080782. - S, pratiksha; T.M., Jamie (2018), " Antacids
",
StatPearls
, StatPearls Publishing, PMID 30252305, retrieved 28 February 2019 - Corbridge, page 1025
- Roncal-Herrero, T; and others. (2009-12-02). "Precipitation of iron and aluminum phosphates directly from aqueous solution as a function of temperature from 50 to 200 °C." Crystal Growing and Design
.
9
(12):5197–5205. DOI: 10.1021/cg900654m. ISSN 1528-7483. - Lagno, F; and others. (2005). "Synthesis of hydrated aluminum phosphate, AlPO 4 1.5H 2 O (AlPO4-H3), by controlled reaction crystallization in a sulfate medium." Industrial and Engineering Chemical Research
.
44
(21):8033–8038. DOI: 10.1021/ie0505559. ISSN 0888-5885.
Reviews
Several reviews about Aluminum Phosphate preparations:
- “... I have been taking this drug for a long time, it helps me cope with heartburn and nausea after eating, and does not cause side effects”;
- “...I always have this medicine in my medicine cabinet, I drink it periodically, as the doctor prescribed. Recently I was poisoned by something, so the medicine even helped me here”;
- “... I like the medicine, improvements come as soon as I start taking it. I take it for reflux, stomach and intestinal problems. The only thing is that it can cause constipation, for such a case I always have a laxative with me.”
Related compounds
AlPO4·2H2O dihydrate occurs as the minerals variscite and metavariscite. Aluminum phosphate dihydrate (variscite and metavariscite) has a structure that can be considered as a collection of tetrahedral and octahedral units of phosphate anions, aluminum cations and water. Al 3+ ions are 6-coordinate and PO 4 3- ions are 4-coordinate.
A synthetic hydrated form of AlPO 4 1.5H 2 O is also known.
Source
| Safety instructions | |||
Please note the labeling exemptions for drugs, medical devices, cosmetics, food and animal feed.
| |||
| To the extent possible and generally accepted, SI units are used. Unless otherwise stated, the data given refers to standard conditions. |
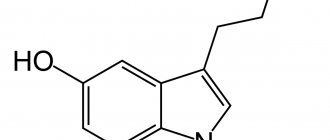
Aluminum orthophosphate
, also known as
aluminum phosphate
, is an aluminum salt that forms orthorhombic crystals. In this regard, aluminum appears in the form of a threefold positively charged cation (Al 3+ before) connected to the phosphate anion (PO 4 3- ) in the electrostatic interaction of the stands.