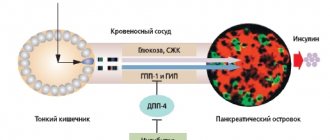
This form of the disease is the most common and occurs in 80–90% of diabetics. Type 2 diabetes mellitus occurs due to insulin resistance - a decrease in the sensitivity of body cells to insulin produced by the pancreas. Risk factors for both adults and children include overweight and obesity, lack of physical activity, poor nutrition and heredity.
Causes of type 2 diabetes:
- genetic predisposition (if the disease is detected in the parents, then the risk of developing it in the child is 40%);
- unhealthy diet (an unbalanced diet leads to disruption of carbohydrate metabolism in the body);
- overweight (visceral obesity, when fat is deposited in the waist and around the abdominal organs);
- physical inactivity (sedentary work, limited mobility due to somatic diseases);
- arterial hypertension;
- inflammatory diseases of the pancreas.
Symptoms of type 2 diabetes mellitus
Signs of the disease are:
- constant thirst;
- severe itching of the skin and mucous membranes;
- weight gain;
- frequent urination;
- drowsiness, increased fatigue;
- slow wound healing;
- numbness of the limbs;
- calf muscle cramps;
- constant feeling of hunger;
- vision problems.
Most often, the disease is found in people over 40 years of age who are obese.
Stages of development of type 2 diabetes mellitus:
- Easy. Symptoms are practically not expressed; laboratory tests of urine and blood may show minor deviations.
- Moderate weight. In addition to the alarming test data, there are pronounced symptoms.
- Heavy. A sharp deterioration in the patient's condition with the development of complications.
The danger of type 2 diabetes mellitus is due to the risk of developing metabolic syndrome against the background of increased concentrations of glucose in the blood. This syndrome combines diseases such as arterial hypertension, ischemic heart disease, dysproteinemia, and obesity.
Among the consequences of type 2 diabetes mellitus are:
- hyperosmolar coma;
- lactic acidosis;
- hypoglycemia;
- diabetic nephropathy;
- myocardial infarction;
- stroke;
- diabetic ophthalmopathy;
- deformation or death of skin tissue on the feet;
- miscarriages and congenital malformations of the fetus.
results
Systematic literature review
- Based on the results of the literature review, 27 publications were selected, covering 26 studies that used triple therapy for type 2 diabetes.
- 9 studies (10 publications) were excluded from the network meta-analysis because they compared different drugs belonging to the same pharmacological groups, for example, the effect of treatment with MET + PSM + insulin was compared with the effect of using the same combination.
- 1 study was excluded because it did not include a comparator group.
- Most of the remaining studies were conducted over 24 to 26 weeks (11 of 17 studies).
- Only 5 lasted for a year or longer.
- Of the 17 studies that were included in the network meta-analysis, 3 were at high risk of bias. In 9 of them it was defined as unclear, and in 5 studies as low.
- Only 2 studies were not subsidized.
- In general, the key characteristics of triple therapy differ and, as a result, their comparability may be limited.
- Study participants were recruited from 2002 to 2011. Most of the testing was carried out in accordance with international standards.
Main research characteristics:
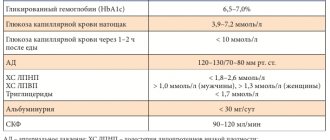
- Participants are adult patients with glycated hemoglobin levels from 7%
- In each study, the standard deviation of HbA1c in the study group compared to the control group was of greatest interest.
- Study power was determined by examining this variable, but Rosenstock et al did not calculate the required sample size.
Initial characteristics:
- Baseline characteristics were generally similar across the pool of studies using the triple treatment, but there was some variability that could lead to heterogeneity between studies and treatments within the network meta-analysis.
- In such studies, the subsequent potential effect of modifiers varied depending on the treatment regimen;
- The initial level of glycated hemoglobin ranged from 8.1% to 10.3%.
- The duration of type 2 diabetes varied from 5 to 10 years.
- Body mass index - from 27 to 35 kg/m2.
- The second most important outcome in most studies was hypoglycemia. The frequency of its detection varies in different studies.
Results of therapy effectiveness:
- Most studies show 2 indicators of the effectiveness of therapy: changes in glycated hemoglobin levels and changes in body weight.
- Seventeen RCTs containing data on glycated hemoglobin levels at 6 months after treatment initiation were selected for inclusion in the network meta-analysis ( N = 9, 144).
- The parameter for selecting 16 RCTs for network meta-analysis was the change in body weight of the subjects after 6 months from the start of treatment (N=8,341).
- Network models were tested for data consistency.
- When directly and indirectly comparing the results, differences in both parameters: HbA1c (p=0.996) and body weight (p=0.431) were not identified.
Comparison of the effectiveness of three-component and two-component therapy:
Representatives of all classes of drugs included in the network meta-analysis, in combination with basic therapy, provided a more significant and clinically significant (> 0.3%, > 3.3 mmol/mol) reduction in glycated hemoglobin compared with two-component therapy, including only MET + PSM.
- The only exception is the three-component regimen: MET + TZD + DPP-4, which resulted in no significant differences compared with the results of basic therapy.
- According to the research results, when taking combinations of MET + TZD and PSM + DPP-4-with any comparison drugs, a statistically significant difference in the change in the level of glycated hemoglobin was not obtained.
- Only the use of SGLT2 and GLP-1 receptor agonists (in combination with MET + PSM) led to a significant decrease in body weight compared with two-component basal therapy (standard deviation: −1.76 kg; 95% CI: −2.74 to −0.78 kg and standard deviation: −1.55 kg; 95% CI: −2.34 to −0.74 kg, respectively)
Results of a two-component treatment regimen including PSM + DPP-4 (standard deviation −2.89 kg; 95% CI: −4.20 to −1.59 kg and standard deviation: −2.60 kg; 95% CI: −3.79 to −1.42 kg respectively); and results of MET + TZD dual therapy (SD: −1.833 kg; 95% CI: −3.36 to −0.30 kg and SD: −1.59 kg; 95% CI: −2.95 to −0.22 kg, respectively).
Against the background of the use of three-component treatment regimens MET + PSM + TZD (standard deviation: 3.5 kg; 95% CI: 2.3 - 4.6 kg) and MET + PSM + insulin (standard deviation: 2.5 kg; 95% CI: 1.5 to 3.4 kg) , in contrast to the baseline, there was a significant increase in body weight.
Comparison of the effectiveness of different options for three-component therapy:
- When comparing the results of using different regimens of three-component therapy, there was no significant difference in the change in glycated hemoglobin in each of the comparison groups.
- Only 2 comparison combinations demonstrated equal efficacy: MET + PSM + insulin and MET + PSM + GLP-1 receptor agonist (SD: −0.01% (0.1 mmol/mol), 95% CI: −0.32 to 0.30% (− 3.5 to 3.3 mmol/mol).
- Based on the results of applying most three-component schemes, significant differences were identified between them in relation to changes in the weight indicator.
- As a result of the use of combinations with MET + PSM, DPP-4-i, TZD and insulin, a statistically more significant weight gain was noted compared to the results of using three-component regimens: MET + PSM + SGL2-i or MET + PSM + GLP-receptor agonist 1.
- The use of the MET + PSM + TZD regimen led to more significant weight gain compared to the use of MET + TZD + DPP-4, as well as MET + PSM + DPP-4.
Analysis of the likelihood of side effects:
- Hypoglycemia was the only side effect reported in most studies.
- 10 RCTs were selected for inclusion in the network meta-analysis based on the presence of this criterion (N=4, 458).
- Data on the results of using three-component therapy: MET + PSM + sodium-glucose cotransporter type 2 inhibitor were available only at the control time point, 12 months after the start of treatment. Data on treatment outcome after 6 months were not available, and therefore this triple therapy option was not included in the network meta-analysis.
- The incidence of all adverse events in the studies, including serious and severe hypoglycemia, was not systematically recorded and, for this reason, a network meta-analysis of their incidence cannot be presented in more detail.
Comparison of the likelihood of side effects when using three-component and two-component treatment regimens:
- The use of combinations of MET + PSM + TZD, MET + PSM + DPP-4 and MET + PSM + insulin led to an increase in the odds ratio for developing hypoglycemia (1.61; 95% CI [0.90–2.87]), while the use of the combination MET + PSM + GLP-1 receptor agonist did not contribute to a significant increase in OR compared to basic therapy.
Comparison of the likelihood of side effects when using different options for three-component therapy:
- As a result of the use of various options for three-component therapy, no significant difference was found in the odds of developing hypoglycemia. However, as a result of the use of the three-component regimen MET + PSM + GLP-1 receptor agonist, the odds ratio for developing hypoglycemia decreased compared with those using the MET + PSM + insulin regimen (OR 0.44; 95%, CI [0.25-0.76]
Network models were tested for goodness-of-fit, and inconsistency was found between direct and indirect comparisons of hypoglycemia odds (p=0.004). Most of the discrepancies are attributed; Hermansen et al (2007) (tested for inconsistency; p = 0.144 study; Hermansen et al excluded).
Diagnosis of type 2 diabetes mellitus
For examination and therapy, you should contact an endocrinologist. The doctor interviews the patient for complaints, clarifies the medical history (including family history), and conducts a physical examination.
Laboratory diagnostics for type 2 diabetes mellitus comes down to the study of urine and blood (general, biochemical). Tests are also carried out for glucose in the blood, ketone bodies in the urine, glucose tolerance, insulin and C-peptide levels are determined.
Instrumental studies may include ultrasound, angiography, EEG of the brain, etc.
Abbreviations:
| DPP-4-i | dipeptidyl peptidase-4 inhibitors |
| GLP-1-AR | glucagon-like peptide-1 receptor agonists |
| ICPR | Minimal clinically important difference |
| MET | Metformin |
| OSH | Odds Ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized clinical trials |
| SGLT2-i | sodium-glucose cotransporter type 2 inhibitors |
| PSM | Sulfonylureas |
| TZD | Thiazolidinediones |
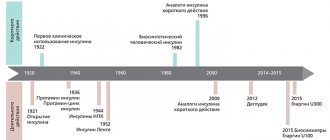
Genetics and type 2 diabetes
In the case of type 2 diabetes, the genetic prerequisites for the development of the disease can be traced especially clearly. 20 genes that are risk factors have already been identified. The sensitivity of peripheral tissues to insulin is reduced due to polymorphism of the ADAMTS9 gene. Glucose tolerance is impaired by increased expression of the TCF7L2 gene product. The KCNJ11 and KCNQ1 genes carry information about the structure of proteins involved in insulin metabolism.
To preliminary assess the risk of developing diabetes, the DQA1, DQB1 and DRB1 genes and some alleles of HLA-DR3 and HLA-DR4 are studied. A genetic study to assess the risk of developing diabetes “Carbohydrate metabolism” is carried out in medical genetics.
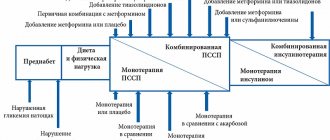
Principles of therapy for type 2 diabetes mellitus
Treatment includes:
- diet (consistent with your doctor);
- taking medications (stimulating the production of insulin, increasing the sensitivity of cells to that hormone, as well as drugs containing insulin);
- regular physical activity.
Diet therapy for type 2 diabetes includes:
- exclusion of flour and sweets;
- reducing carbohydrate intake;
- minimizing fat;
- fractional meals.
A complete list of permitted products can be obtained from an endocrinologist.
Discussion
- According to the results of the review and network meta-analysis, the addition of a third class of drugs to the basic combination of MET + PSM is a statistically and clinically more effective tactic (upper confidence limit greater than the minimum clinically important difference of 0.30%, 3.3 mmol/mol) in reducing glycated hemoglobin compared with two-component therapy.
- Only the use of the combination of MET + TZD + DPP-4 did not lead to a more significant decrease in the level of glycated hemoglobin compared to basic therapy.
- As a result of comparison of different three-component regimens, it turned out to be difficult to identify any difference between them with respect to the effectiveness of achieving the target level of glycated hemoglobin. The results of using all three-component schemes fall within the 95% confidence limits.
- Only in one case, when comparing the effectiveness of the MET + PSM + GLP-1 receptor agonist and MET + PSM + insulin regimens containing the same CI criteria within 0.3% (3.3 mmol/mol), was the statistical error so large that that it was not possible to draw a general conclusion.
- Most clinical guidelines recommend a patient-specific approach when determining the target level of glycated hemoglobin that should be achieved as a result of treatment of type 2 diabetes.
- Regarding triple therapy, guidelines generally recommend insulin as the preferred third drug to the basic MET + PSM combination. Other drugs can be used if the preferred drug cannot be used in a given patient due to contraindications, individual intolerance or financial reasons.
- Some guidelines suggest broadening the scope of goals beyond hyperglycemia control alone and combining multiple goals, including achieving glycated hemoglobin targets, weight loss, and monitoring potential cardiovascular outcomes.
- When assessing the level of glycated hemoglobin and changes in body weight at a critical point in time, the combinations of MET + PSM + sodium-glucose cotransporter type 2 inhibitor and MET + PSM + GLP-1 receptor agonist showed greater effectiveness in reducing both indicators compared to the baseline treatment regimen.
- While other drug combinations appear to be more effective in reducing glycated hemoglobin levels, they did not show any benefit in terms of weight loss (MET + PSM + TZD) compared with the basal regimen, or any difference in weight gain. in weight (MET + PSM + DPP-4, MET + PSM + insulin and MET + PSM + TZD).
- When using the combination of MET + PSM + insulin, the likelihood of developing hypoglycemia was higher than when using MET + PSM + GLP-1 receptor agonist.
- Overall, the systematic literature review identified only limited data on the long-term safety of treating type 2 diabetes with triple therapy. This is due to the fact that the duration of the longest study was 1 year.
- Some two-component combinations of drugs are of interest from the point of view of the possibility of their long-term use, as data from relevant studies are available.
- It is important to note that different drugs belonging to the same pharmacological group were combined during the analysis. This was done on the assumption that the same mechanism of action would provide them with equal effectiveness. For example, the group of insulins includes a long-acting basal insulin analogue (glargine) and an ultra-short-acting insulin analogue (aspart) - they were combined in the analysis. However, this assumption did not greatly affect the overall result, since comparative studies between the two groups showed that glargine and aspart were equally effective and equally safe (Strojek et al., 2009; Yang et al., 2013).
- Other multiple studies have shown similar results: different drugs that belong to the same pharmacological class and were included in the network meta-analysis are characterized by similar efficacy and safety; DPP-4-i (Deacon, 2011), GLP-1 receptor agonist (Rigato & Fadini) and TZD (Norris, Carson & Roberts)).
- Data were available on only one triplet therapy, including a sodium-glucose cotransporter type 2 inhibitor, and information on other combinations was not assessed.
The studies included in this meta-analysis had a number of limitations that needed to be taken into account when assessing its results:
- For most studies, power calculations were performed only to assess changes in glycated hemoglobin levels.
- Short duration (24 to 30 weeks), network meta-analysis results are based on these time points.
- Small number of participants.
- Few reported side effects.
In view of this, it was difficult to draw a conclusion regarding the possibility of side effects with long-term use of triple therapy. In addition, it was not always clear whether data on them were obtained but not documented, or whether such cases did not occur during the study.
Conducting network meta-analyses that include indirect comparative analyzes may introduce statistical bias. For this reason, a random-effects model was used to account for between-study heterogeneity in assessing treatment effectiveness.
-Data obtained using the direct and indirect methods were also checked for consistency to ensure that the results of the network meta-analysis were consistent with the results of the original studies included in it.
There are limitations associated with the availability of information, since only 3 studies did not include the MET + PSM regimen as a basic dual therapy.
The network model of the probability of hypoglycemia revealed some discrepancy between the results of direct and indirect comparisons.
Differences in the incidence of hypoglycemia may be due to a number of reasons. In some studies, if hypoglycemia was recorded, the dose of drugs was adjusted.
In the network meta-analysis, most of the identified inconsistencies were attributed to 1 study conducted by Hermansen et al (2007). The number of reported cases of hypoglycemia varies among studies, and Hermansen et al (2007) did not find any such cases. This finding may be a consequence of the lower threshold for hypoglycemia compared to other studies.
The number of studies providing information on the incidence of hypoglycemia is insufficient to estimate the likelihood of this complication; this may also lead to increased statistical error.