Symptoms
In the initial stages, the symptoms of pseudomembranous colitis may not be very varied - usually abdominal pain and diarrhea. As the disease progresses, the number of symptoms increases, so the following appears:
- Regular diarrhea, which debilitates the patient, greatly reduces his quality of life.
- Change in color of stool. They take on a green tint. They also often contain admixtures of mucus and blood.
- Increased amount of urine.
- Dehydration - due to constant diarrhea.
- Increased body temperature, convulsions.
- Weakness, fatigue, headaches.
- Problems with appetite - often the patient simply cannot eat.
- Bloating, cramping pain - as a rule, they are localized on the left side.
- Increased heart rate, decreased blood pressure.
Obviously, such symptoms of pseudomembranous colitis are not specific - they can also occur with other gastrointestinal diseases, so an accurate diagnosis will require a comprehensive, very thorough examination.
Etiology and pathogenesis
Clostridium difficile is a spore-forming, anaerobic microorganism first described in 1935 [7] . However, the role of Clostridium difficile toxins in the development of pseudomembranous colitis was established only in 1978 [6] . The microorganism does not always cause the development of the disease (since there are strains that do not produce toxins), and its severity can vary from mild diarrhea, without signs of colitis, to life-threatening conditions.
The pathogenesis of the disease includes the following stages
- disruption of the normal microflora of the colon (normal stool contains more than 500 different bacteria in a concentration of 10 to 12 degrees per gram);
- colonization of the colon with toxin-producing strains of Clostridium difficile;
- production of toxins (A and B);
- inflammation and damage to the mucous membrane of the colon (to the point of necrosis of the epithelium and formation of membranes in severe cases).
Most often, the development of the disease is caused by the use of clindamycin, linkamycin, cephalosporins and protected penicillins [3] . In addition to recent (usually no later than 2 months) use of antibiotics, the risk of developing infection is increased in persons over 60 years of age, inpatients, patients with weakened immunity, renal failure, cancer, and in persons receiving antisecretory therapy.
Danger of disease
If a person does not receive timely treatment, and signs of dehydration and intoxication increase, complications from pseudomembranous colitis can be serious. There are recorded cases of death due to the fact that complications of such colitis can cause cardiac arrest.
Complications also include perforation of the intestinal wall, peritonitis, renal failure and other diseases, which in themselves can also lead to death.
Literature
- Alfa MJ, Dul T., Beda G. Survival of incidence of Clostridiulm difficile infection in Canadian hospitals and diagnostic approaches// J. Clin. Microbiol. 1998. V. 36. P. 2076-2080.
- Barbut F., Kajzer C., Planas N., Petit JC Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for the diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol 1993;31:963-7.
- Bartlett JG ANTIBIOTIC-ASSOCIATED DIARRHEA N Engl J Med, Vol. 346, No 56: 334-339.
- Dallal MR Fulminant Clostridium difficile: An Underappreciated and Increasing Cause of Death and Complications. Ann Surg. March 2002; 235(3): 363–372.
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol 1997;92:739-50.
- George RH, Symonds JM, Dimock F., et al. Identification of Clostridium difficile as a cause of pseudomembranous colitis. BMJ 1978;1:695.
- Hall IC, O'Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 1935; 49:390-402.
- Jacobs J., Rudensky B., Dresner J., et al. Comparison of four laboratory tests for diagnosis of Clostridium difficile–associated diarrhea. Eur J Clin Microbiol Infect Dis 1996;15:561-6.
- Johnson S, Kent SA, O'Leary KJ, et al. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann Intern Med 2001;135:434-8
- Kelly CP, Pothoulakis C, LaMont JT Clostridium difficile colitis. N Engl J Med 1994;330:257-62
- Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev/Med 1998;49:375-90.
- Kreutzer EW, Milligan FD Treatment of antibiotic-associated pseudomembranous colitis with cholestyramine resin. Johns Hopkins Med J 1978;143:67-72.
- Malnick S., Zimhony O. Treatment of Clostridium difficile–Associated Diarrhea. Ann Pharmacother 2002;36:1767-75.
- Manabe YC, Vinetz JM, Moore RD, Merz C., Carache P., Bartlett JG Clostridium difficile colitis: an efficient clinical approach to diagnosis. Ann Intern Med 1995;123:835-40.
- Mylonakis E., Ryan ET, Calderwood SB Clostridium difficile–associated diarrhea. A review. Arch Intern Med 2001;161:525-33.
- Staneck JL, Weckbach LS, Allen SD, et al. Multicenter evaluation of four methods for Clostridium difficile detection: immunoCard C. difficile, cytotoxin assay, culture, and latex agglutination. J Clin Microbiol 1996;34:2718-21.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhea and colitis. Lancet 1983;2:1043-1046.
- Wenisch C., Parschalk B., Hasenhundl M., Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996;22:813-8.
- Young G., McDonald M. Antibiotic-associated colitis: why do patients relapse? Gastroenterology 1986;90:1098-1099.
Causes
The main cause of pseudomembranous colitis is a violation of the intestinal microflora. And it occurs against the background of various conditions and provoking factors, for example:
- Long-term (or uncontrolled) use of certain medications. This effect is caused by antibiotics, cytostatics, etc.
- Disturbances in the electrolyte and protein metabolism of the body.
- Malignant tumors that reduce the body's protective functions.
- Weakened immunity after major surgery.
- Constant contact with patients who already suffer from such colitis.
Other causes of pseudomembranous colitis are also found if we are talking about a weakened immune system with a simultaneous disruption of the intestinal microflora.
Diagnostics
Diagnosis of pseudomembranous colitis begins with examination of the patient and collection of anamnesis. Depending on the complaints the patient presents, the examination is carried out either by a gastroenterologist or a proctologist.
Diagnostics includes taking a smear from the anus, colonoscopy, sigmoidoscopy, endoscopy, abdominal ultrasound (or MRI), and radiography. It is not necessary that all studies be carried out at once - in some cases, diagnostic methods can be expanded or, conversely, used in limited quantities.
The patient is also given a general blood test and stool to examine its composition.
Treatment
If the situation is not advanced, the patient is treated with conservative methods - first of all, the fluid supply in the body is restored. Treatment for pseudomembranous colitis also includes:
- Symptomatic treatment. It involves eliminating the symptoms of intoxication, restoring water balance, electrolyte and protein metabolism. Anti-diarrhea medications are not used because they can cause serious side effects.
- Special diet for pseudomembranous colitis. It is immediately therapeutic, and then supportive. For example, at the maintenance stage it is necessary to exclude pickled, fried, smoked, fatty foods, white bread and soda. The doctor will provide the patient with a complete list of prohibited and permitted products.
- Medicines to eliminate the cause of the disease (antibacterial drugs).
- Correction of dysbiosis: prescribing bacterial drugs.
- Surgical intervention. It is used if serious complications begin or conservative methods do not work. During surgery, part of the intestine may be removed, and the intestine itself may be brought into the abdominal cavity - the type of operation depends on the situation.
We recommend entrusting the checking of symptoms and treatment of pseudomembranous colitis to the specialists of JSC "Medicine" - first you need to seek advice from a gastroenterologist or proctologist. Remember that this is an extremely dangerous disease that is much easier to treat in the early stages. The longer the problem develops, the higher the risks of dangerous complications.
Pseudomembranous colitis
Every Medical Advance Has a Price Richard K. Root
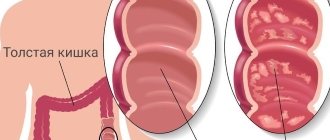
| On the left are white-yellow plaques in the sigmoid colon, on the right are confluent plaques forming pseudomembranous fields in the colon |
- Etiology
Clostridiulm difficile (CD) bacteria are quite large (from 0.5-1.9 to 3.0-16.9 µm) motile gram-positive bacteria belonging to the group of obligate anaerobes (genus Clostridiulm), which form oval subterminal spores under unfavorable conditions, which are resistant to heat and capable of long-term (several years) survival under aerobic conditions. The temperature optimum for growth of vegetative forms is 30-37°C. The most important pathogenicity factors for CD are enterotoxin A and cytotoxin B.
- Epidemiology
KDs are widespread in nature and have a ubiquitous distribution, since they are permanent inhabitants of the intestines of many animal species (domestic and wild), and in some cases can be found in the feces of healthy people of various age groups, including newborns. As a result of soil contamination, CDs can be detected on various environmental objects, which poses a particular problem in medical institutions. Despite the fact that KDs are not pathogenic bacteria, nevertheless, under certain conditions they are capable of causing diseases such as antibiotic-mediated diarrhea (AMD) and pseudomembranous colitis (PMC). Detection of CD on environmental objects in hospitals poses a serious threat of infection to patients. Although the risk of infection through direct contact with a bacterial pathogen or a patient is usually low, prolonged contact can lead to the development of clinically manifest forms of CD infections (CDI). In the literature, there are numerous descriptions of nosocomial outbreaks of AOD in departments of various profiles [9], including geriatric, orthopedic, surgical, etc., which allows us to speak of CDI as a nosocomial infection. Theoretically, an outbreak of CDI can be registered in departments of any profile, but observations show that patients in surgical departments and intensive care units are at the greatest risk of contracting CDI [7, 10]. The following pattern is quite clearly visible: the more intensively invasive methods of diagnosis and treatment and aggressive therapy are used, the higher the risk of developing CDI. As epidemiological studies show, the proportion of nosocomial infections caused by KD is constantly growing, especially in those countries where the appropriate laboratory and diagnostic facilities have been established [4].
It is quite difficult to control the situation with nosocomial infection of KD patients. Firstly, control over the contamination of environmental objects with KD spores is not carried out in hospitals; secondly, KD spores are resistant to the action of standard disinfectants.
As observations show, from 3 to 6% of healthy people are carriers of KD. Healthy children in the first year of life, including newborns, are carriers of CD much more often - 30-90%, although the development of MVP in this age group is atypical. In hospitals, the frequency of detection of CD bacteria carriers may be higher. At the same time, bacteria carriers, as a rule, do not have any clinical and laboratory indications of the development of CDI in the anamnesis.
The mere fact of infection of patients with KD is not decisive in the development of CDI. The conditions necessary [4] for the development of ICD, including MVP, are:
- presence of a source of infection;
- oral intake of antibiotics or other groups of drugs that can cause disruption of intestinal microbiocenosis;
- colonization of the colon mucosa by CD and production of exotoxins;
- individual risk factors: age, previous diseases and hospitalizations, duration of the disease.
A critical factor for the development of ICD, including MVP, is a decrease in colonization resistance of the intestine, in particular the colon, as a consequence of impaired microbiocenosis. A feature of the microbial ecology of the large intestine is the absolute dominance of anaerobic bacteria in it, in a ratio of 1000:1 to aerobes and a population density of about 1012 microbial bodies per 1 g of feces. Despite such a high population level of indigenous microflora in the large intestine, this microbiocenosis is easily disrupted, especially under the influence of antibiotics or other external factors, and is restored extremely slowly [1, 2].
| Pseudomembranous colitis (PMC) is a rare but dangerous disease caused by the spore-forming anaerobic microbe Clostridiulm difficile. Despite the fact that the clinical manifestations of MVP are very variable, most often patients experience prolonged diarrhea, intoxication, abdominal pain and leukocytosis, which usually occur during antibiotic therapy |
The most important and most powerful co-factor contributing to the development of CDI is the use of antibiotics. Antibiotic therapy precedes the development of MVP in 60-85% of cases. Despite the fact that CDI can be provoked by almost any antibiotics and/or antimicrobial drugs, including sulfonamides and metronidazole, most often the development of CDI is observed while taking third-generation cephalosporins, clindamycin, ampicillin, amoxicillin with clavulanic acid, and fluoroquinolones. Macrolides and rifampicin rarely act as co-factors in the development of CDI. It should be remembered that neither the dose, nor the frequency, nor even the route of administration of the drug affects the possibility of developing CDI. There have been cases where even a single administration of an antibiotic led to the development of AOD and MVP.
In addition, cases of the development of ICD, including MVP, have been described when using chemotherapy, antineoplastic drugs, immunosuppressive therapy, gold drugs, nonsteroidal anti-inflammatory drugs, antidiarrheal drugs, and antipsychotics. Disruption of intestinal microecology, accompanied by colonization of CD, occurs during major abdominal operations, long-term use of nasogastric tubes and enemas, in patients in intensive care units and intensive care units, in renal failure and some other conditions.
Practice shows that CDI is most often detected in surgical hospitals, especially in patients who have undergone intestinal surgery. This circumstance can have only one explanation: in addition to the fact that extensive surgical operations themselves can contribute to the development of CDI, such patients in more than 90% receive broad-spectrum antibiotics, both for treatment and prevention. Yet approximately 10-11% of surgical patients develop CDI without prior antibiotic use.
Studies by Canadian authors [3] have shown that the most serious problem of AOD is in hospitals with more than 200 beds, in which the incidence rate of CDI ranges from 30.8 to 40.3 cases per 100 thousand patients. According to other observations [10], MVP develops in outpatients when receiving oral antibiotics with a frequency of one to three cases per 100 thousand patients, and among hospitalized patients, the incidence of MVP is one in 100 (depending on the profile of the hospital).
Thus, many researchers conclude that KD is the leading clinically significant pathogen responsible for the development of nosocomial diarrhea, which accounts for 20 to 45% of all hospital-acquired diarrhea [8].
- Pathogenesis
Although CDs can be detected in the stool of healthy individuals, there is convincing evidence that these microorganisms are not capable of long-term survival in the intact normal intestinal microecosystem. A factor contributing to the colonization of the colon by CD is the deep inhibition of the anaerobic part of the indigenous intestinal microflora.
The leading factors in the pathogenicity of KD are toxic substances produced by KD: toxin A (TA) and toxin B (TB), which in vivo exhibit a synergistic effect [6]. TA is a powerful enterotoxin with cytotoxic activity, causing disruption of the barrier function of the intestinal mucosa due to damage to epithelial cells and activation of fluid secretion into the intestinal lumen. TB is a 1000 times more potent cytotoxin than TA, but its cytotoxic effect is due to disruption of the polymerization of intracellular actin filaments. Picogram TV can have a cytopathic effect. The morphological changes in the mucosa detected in the colon are caused by the action of toxins only, since CDs themselves do not have invasive properties and, as a rule, do not penetrate into the submucosal layer. The extent and depth of morphological changes detected in the colon determine the severity of the infectious process.
Despite the fact that CDI most often develops after treatment with antibiotics, nevertheless, the interaction between the body's protective factors, the external environment and CD remains largely poorly understood, due to which many aspects of the pathogenesis are still unclear.
- Clinic
The spectrum of clinical manifestations of CDI varies widely: from asymptomatic carriage and self-limited diarrhea to severe colitis. Since MVP is an extreme form of ICD with a possible recurrent course, often an unfavorable prognosis and requires particularly complex treatment, doctors pay the most attention to this form of the disease. In table Table 1 presents risk factors for the development of colitis caused by KD.
Table 1. Risk factors for developing colitis due to KD
|
The average age of patients is 58-60 years, although the development of CDI does not fundamentally depend on the age of patients [5]. The exception is newborns and children under one year of age, in whom the development of CDI is atypical, and this despite the fact that the frequency of detection of toxigenic strains of CD in their intestines is much higher than in adults. The paradox of this situation is explained by the fact that in this age group there are no receptors for KD toxins in the intestinal epithelium.
AOD is characterized by the development of diarrhea syndrome (DS) against the background of antibiotic therapy, while the general condition of patients, as a rule, does not change. The body temperature remains at the original level, minor abdominal pain may occur, and the stool is moderately watery. Diarrheal syndrome is short-lived and resolves within one to three days after stopping antibiotic therapy without additional treatment. It is quite difficult to establish the true number of patients with AOD caused by KD, since this condition is mild, without severe dehydration and, as a rule, ends with spontaneous recovery.
The clinical picture of MVP is very variable, since this disease complicates the course of the main pathological process. Typical symptoms of MVP are loose stools, abdominal pain and fever. The severity of these signs can vary widely. MVP develops, as a rule, either directly against the background of antibiotic therapy, or seven to ten days (in rare cases later) after cessation of antibiotic therapy.
Table 2. Risk factors for the development of recurrent MVP
|
The clinical picture of MVP is dominated by DS, which in some cases is the only manifestation of the disease. DS, being the most constant clinical sign of MVP, is detected in 100% of cases at the onset of the disease. In some cases, the manifestation of the disease may begin with fever. The frequency of bowel movements per day reaches five or more times, sometimes reaching 20-30. The stool is usually watery and of small volume, but given the frequency of bowel movements, patients may develop fluid and electrolyte disorders of varying severity. Diarrhea is persistent and can persist for up to eight to ten weeks. In some cases, stool disorder may be intermittent, when diarrhea is replaced by formed stools that persist for one to two days. Often the stool contains an admixture of mucus, while an admixture of blood is not typical. Vomiting is quite rare and is detected in later stages of the disease, indicating the severity of its course.
In fact, simultaneously with DS, patients experience abdominal pain of varying intensity, predominantly of a spastic nature, which intensifies with palpation of the abdomen. Most often, the pain does not have a clear localization and is determined along the intestine.
In most cases, the body temperature of patients with MVP remains at febrile levels, however, in recent years, cases of the disease in which hectic fever exceeding 40°C are recorded have become more frequent.
Characteristic of MVP is a fairly pronounced leukocytosis of peripheral blood, reaching 15x109/l, and in some cases even leukemoid reactions are detected, in which the number of leukocytes can reach 40x109/l. There are isolated observations when MVP develops in patients against the background of leukopenia. As a rule, leukopenia is recorded in patients receiving chemotherapy for malignant tumors. MVP in such patients is extremely severe and often has a fulminant course with the development of bacteremia. It is the fulminant course of MVP that poses the greatest difficulty in terms of diagnosis due to the unusualness of the clinical symptoms detected, since in this case there is a combined lesion of the large and small intestine.
The fulminant course of MVP is characterized by rapid progression of the process. DS, which is key for AOD in fulminant MVP, may be absent. Almost half of the patients experience constipation and signs of intestinal obstruction. In such patients, signs of an “acute abdomen” are detected; the fever is above 38.4°C [9]. Computed tomography of the abdomen reveals ascites and significant thickening of the colon wall. Despite the clear clinical signs of an “acute abdomen,” free air in the abdominal cavity is not detected. A feature of the management of such patients is that basic drug therapy is ineffective and radical surgical intervention (subtotal colectomy) is required. Mortality in fulminant MVP reaches 58%.
Given the long-term and persistent nature of diarrhea, patients with MVP often exhibit severe electrolyte disturbances (hypokalemia), hypovolemia, decreased plasma albumin levels, the development of edema up to anasarca, and hypotension.
MVP can be complicated by the development of toxic megacolon, perforation of the colon with the development of peritonitis, and infectious-toxic shock.
Recurrent MVP is observed in 20% of patients with a primary diagnosis after standard antibiotic therapy. For patients who have at least one relapse, the risk of recurrent MVP increases to 45-68%. The mechanism of formation of the recurrent course of MVP is still not fully understood. It is believed that the main reason is incomplete sanitation of the intestines from KD spores, although the possibility of reinfection cannot be ruled out.
Risk factors for the development of recurrent MVP are presented in Table. 2.
- Diagnostics
Diagnosing ICD is a complex problem. Isolation of the pathogen, although it seems important, is of secondary importance due to the slow growth of bacteria. The most important method for verifying the diagnosis of MVP is the detection of CD toxins in the stool. Toxigenic KD strains that cause the development of MVP usually produce both toxins, but the “gold standard” in diagnosing MVP can be the detection of TV when testing a cell culture, which is the most sensitive method. The proposed commercial ELISA kits for the detection of toxins A and B, unfortunately, are inferior in specificity and sensitivity to the cytotoxic test. In recent years, PCR methods have been developed as an alternative to the cytotoxic test. As many studies show, latex agglutination, used to diagnose CDI, today cannot be recommended as a “basic” diagnostic method, since it has a fairly low sensitivity.
Endoscopic methods have not lost their diagnostic value. Pathological changes are localized mainly in the distal part of the colon, and to identify them it is enough to perform sigmoidoscopy, however, in one third of patients, pathological changes are localized only in the proximal part of the colon; in this case, a colonoscopy is required. The small intestine can also be involved in the pathological process, but this fact is revealed only at autopsy. During sigmoidoscopy and colonoscopy, diffuse hyperemia and swelling of the intestinal mucosa with thickening of the intestinal wall is noted, although a slight inflammatory reaction is detected in the intestinal wall, and an accumulation of lymphocytes is detected in the submucosal layer. On the surface, characteristic yellowish-white fibrinous plaques with a diameter of 2 mm to 2 cm or more are found (see figure), covering ulcers of the intestinal mucosa. These plaques may coalesce to form pseudomembranous fields. Pseudomembranes are found between the rectum and the left flexure of the colon, but the transverse colon may also be affected. Histological examination shows that plaques consist of fibrin, mucin, desquamated epithelial cells, destroyed leukocytes and microbial flora of the large intestine.
- Treatment
The nature and scope of therapeutic measures taken in patients with ICD is determined by the clinical variant of the disease.
Asymptomatic carriage, as a rule, does not require special treatment methods.
When a diagnosis of MVP is made, treatment begins immediately. First of all, if the disease has developed during antibiotic therapy, it must be discontinued.
The therapy carried out by patients with PMC has two main goals: firstly, it is necessary to stop the inflammatory process in the intestines and, secondly, to sanitize the intestines from CD spores [4, 10].
Despite the fact that antibiotics most often cause the development of CDI, it is antibacterial therapy that is an integral part of the standard basic therapy for MVP, which limits intestinal colonization with CD and stops inflammatory changes in the intestine.
KDs in vitro exhibit varying sensitivity to antibiotics, but the most stable sensitivity is observed to vancomycin and metronidazole. A prerequisite for etiotropic therapy in patients with MVP is enteral administration of antibiotics, since their parenteral administration does not create sufficient concentration in the intestine and sanitization of the body does not occur.
Although vancomycin is usually prescribed at a dose of 500 mg, studies have shown that a dose of 125 mg four times a day is sufficient to control inflammation. Due to the limited availability of vancomycin, metronidazole is more widely used and is prescribed at a dose of 250–500 mg three to four times daily. Oral metronidazole is in most cases equivalent to vancomycin, which is preferred in severe cases of MVP. The course of antibiotic treatment is 10 days. If oral administration of drugs is not possible, they are administered through a nasogastric tube. In rare cases, with severe MVP, a combined administration of vancomycin (per rectulm) and metronidazole (intravenously) is possible, but it should be remembered that intravenous administration of drugs is less effective.
Bacitracin can also be used as basic antibacterial therapy, but due to the variability and instability of the pharmacological effect on KD, preference is given to metronidazole and vancomycin.
| Pathomorphological changes in MVP are detected mainly in the colon and are characterized by diffuse hyperemia, as well as swelling of the intestinal mucosa with thickening of the intestinal wall and the formation of characteristic fibrinous deposits in the form of yellowish-white plaques |
Basic antibacterial therapy contributes to a fairly rapid relief of the clinical manifestations of the disease: normalization of temperature occurs, as a rule, within 24–48 hours, and the frequency and character of stool is restored on the 1st–13th day (an average of 4.5 days).
There were cases when, when antibacterial therapy was ineffective in patients with MVP, normal human immunoglobulin (IG) was used for intravenous administration at a dose of 200-300 mg/kg body weight. The basis for prescribing this drug was control studies that showed that IG drugs contain antibodies against TA and TV at a concentration of 0.4-1.6 mg/ml IgG. During therapy, patients experience rapid cessation of diarrhea, relief of abdominal pain and normalization of temperature.
Unfortunately, no matter what course of antibiotics we use, none of them guarantees complete sanitation of the intestines from CD spores, which poses a threat to the development of a recurrent course of MVP [2]. Preparations based on the non-pathogenic yeast Saccharomyces boulardii (SB) have been proven to be highly effective for sanitizing the body from CD spores. Based on a large number of observations, we can conclude that the therapeutic effect of SB is manifested both when administered in combination with antibiotics, and when conducting an independent course after antibiotic therapy. The mechanism of action of SB is not fully understood. It has been established that they inhibit the growth of CD, reduce the formation and accumulation of cytotoxin in the intestine, and inhibit the intestinal effects of toxins due to proteolysis of the toxins themselves and inhibition of the binding of toxins to receptors. The highest clinical effectiveness of SB was established mainly in patients with recurrent MVP. As observations show, SB should not be used during the first episode of MVP. SB is prescribed 1 g per day for four weeks.
The effect of the use of enterosorbents and ion exchange resins (for example, cholestyramine, cholestipol), despite their ability to bind CD toxins, is assessed by many clinicians as very modest.
In patients with a fulminant course of MVP, basic therapy is often ineffective, so in such cases surgical treatment is performed [9].
The absolute indication for surgical treatment (colectomy with ileostomy) is the presence of signs of peritonitis. Literature 1. Bondarenko V. M., Uchaikin V. F., Murashova A. O., Abramov N. A. Dysbiosis. M., 1995. 2. Malov V. A., Bondarenko V. M., Pak S. G. The role of Cloctridiuln difficile in human pathology // Journal. microbiol.1996. No. 1. pp. 91-96. 3. Alfa MJ, Dul T., Beda G. Surveillance of incidence of Clostridiulm difficile infection in Canadian hospitals and diagnostic approaches// J. Clin. Microbiol. 1998. V. 36. P. 2076-2080. 4. Bartlett JG Clostridiulm difficile: clinical consideration // Rev. Infect. Dis. 1990. V. 12. Sulpl. 2. P. S243-S251. 5. Bolton RP, Thomas DF Pseuldomembranouls colitis in children and adults // Br. J. Hosp. Med. 1986. V. 35. P. 37-42. 6. Branka JE et al. Early fulnctional effects of Clostridiulm difficile toxin A on hulman colonocytes // Gastroenterology. 1997. V.112. No. 6. P. 1887-1894. 7. Bulstrode NW et al. Clostridiulm difficile colitis after aortic sulgery // Eulr. J.Vasc. Endovasc. Sulg. 1997. V. 14. No. 3. P. 46-84. 8. Hulghes JM, Jarvis WR Nosocomial gastrointestinal infection // In RP Wenzel (ed) Prevention and control of nosocomial infection. The Williams & Wilkins Co. Baltimore, 1987, pp. 405-439. 9. Medich DS, Lee K, Simmons RL et al. Laparotomy for fulminant pseuldomembranouls colitis // Arch. Sulg. 1992. V.127. No. 7. P. 847-852. 10. Stergachis A. Perara DR, Schnell MM et al. Antibiotic-associated colitis // West. J. Med. 1984. V. 40. P. 217-219.
Historical reference
MVP was first described by the American surgeon J. Finney in 1893 in a young woman who was operated on for a tumor of the pyloric part of the stomach. The patient developed severe diarrhea resulting in death on the 15th day. At autopsy, diphtheritic membranes were found in the intestines, which, in fact, served as the basis for the introduction of this term. In the “pre-antibacterial era,” PMC was detected in patients extremely rarely and the diagnosis was made only at autopsy. With the introduction of antibiotics into everyday clinical practice, the problem of MVP has worsened, as the number of such patients has increased sharply.
In 1965, S. Goulstone and V. McGovern, analyzing the anatomical and histological changes found in patients with MVP in the intestine, suggested that this disease is caused by an unknown toxic agent acting locally. In 1969, J. Small found that laboratory animals receiving lincomycin hydrochloride developed a typical pathomorphological picture of PMC, which became a laboratory model for studying PMC in subsequent studies. R. Green in 1974 revealed cytotoxicity in cell culture of the intestinal contents of laboratory animals treated with antibiotics - this suggested that an unknown virus plays a certain role in the development of the cytotoxic effect. The establishment of the dependence of the development of diarrhea and/or colitis on the background of the use of antibiotics served as the basis for the widespread use in clinical practice of the term “antibiotic-mediated diarrhea (colitis)”.
Although I. Hall and E. O'Toole first discovered Clostridiulm difficile in human intestinal contents in 1935, its etiological role in the development of MVP and antibiotic-mediated diarrhea (AOD) was established only in the second half of the 70s.
Prevention
Preventive measures include:
- Responsible and competent use of antibiotics. This should be done only as prescribed by a doctor and in accordance with the instructions. It is extremely dangerous to prescribe such drugs to yourself.
- The use of drugs that restore intestinal microflora after antibacterial therapy.
- Strengthening the immune system on an ongoing basis - maintaining a healthy lifestyle, giving up bad habits.
- Maintain personal hygiene. This includes not using other people’s funds or personal belongings.
There is no specific prevention in this case - you just need to take a responsible attitude towards your own health.