Retinoids (in-depth review)
Acnecutane, Roaccutane, Erase
Acnecutane, Roaccutane, Erase
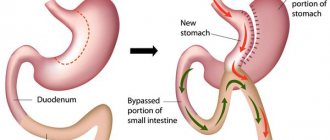
Systemic retinoids contain the active ingredient isotretinoin. This substance has been used in world medicine for almost 40 years and is the gold standard for the treatment of acne and acne.
What kind of substance is this? This is trans-retinoic acid and it very powerfully suppresses the activity of the sebaceous glands.
When does acne occur?
Acne occurs when, under the influence of excessive effects of the hormone testosterone on the sebaceous gland, powerful production of sebum in large quantities is stimulated.
How do systemic retinoids work?
Systemic retinoids act on the sebaceous gland receptors, joining the sebaceous gland receptors, they inhibit the activity of sebum secretion from the gland and acne goes away.
The most frequently prescribed drugs from systemic retinoids (approved in the Russian Federation):
- Roaccutane
- Aknekutan
- Will erase
Comment from cosmetologist, dermatologist Yuliana Shiyan:
When I prescribe one of these drugs, I am asked why this particular drug and what is the difference between retinoids? All these drugs have one substance in common: Isotretinoin. Roaccutane was the very first to appear. This is the original drug, and all other drugs are its *generics. All generics of isotretinoin have their own registration certificates and are approved in Russia.
*GENERIC - approved copy of the original drug
Roaccutane and Sotret practically do not differ from each other either in formula or dose. When assigning them, there is no difference and the choice in favor of one or the other is immaterial. Acnecutane contains the same substance, but its formula is slightly different from Roaccutane and Sotret.
Why is Acnecutane more interesting for a cosmetologist and the patient himself, as a choice for treatment and how to understand which retinoid drug is better?
Isotretinoin is a fat-soluble substance. It is absorbed by the body only in combination with absorbed fats. If you take Roaccutane and wash it down with plain water, you will absorb less than 40% of it. If you take Acnecutan and drink it with plain water, it will be absorbed by the body by about 70%.
When prescribing Acnecutane, the doctor insures patients against their own mistakes. Patients may neglect fatty foods while taking Roaccutane and Sotret, forget to eat anything fatty or fatty foods are forbidden to them (on a diet), and the required dose of the drug must enter the body! Necessary, not a small part of it! Therefore, the drug Acnekutan is more preferable for the patient; a lack of fat when taking it is less likely to affect the outcome of treatment.
Its daily dose is regulated by the doctor, but there is also a cumulative dosage of the drug that the patient’s body must accumulate. It is when it is achieved that we can talk about a high chance of stable remission in the patient.
Due to the fact that Acnecutane is better absorbed, the manufacturer made slightly less active ingredient in one dose, which means: The working dose is 20 mg. Roaccutane corresponds to the working dose of Acnecutane with 16 milligrams of Isotretinoin.
The conclusion is simple: if less isotretinoin accumulates in the body, then the risks of side effects are also reduced. This is very good for the patient!
For ardent supporters of all original and original drugs, Roaccutane is recommended. But all other retinoids will be useful for you, provided that they are prescribed by a dermatologist, and you, in turn, comply with all the conditions for taking retinoids.
Roaccutane
Use during pregnancy and breastfeeding
Pregnancy is an absolute contraindication for Roaccutane therapy.
If pregnancy occurs, despite warnings, during treatment or within a month after the end of therapy, there is a very high risk of giving birth to a child with severe malformations. Isotretinoin is a drug with a strong teratogenic effect. If pregnancy occurs during a period when a woman takes isotretinoin orally (at any dose and even for a short time), there is a very high risk of giving birth to a child with developmental defects.
Roaccutane is contraindicated in women of childbearing potential unless the woman's condition meets all of the following criteria:
- she must suffer from severe acne that is resistant to conventional treatments;
- she must certainly understand and follow the doctor’s instructions;
- she must be informed by the doctor about the danger of pregnancy during treatment with Roaccutane, within one month after it, and urgent consultation if pregnancy is suspected;
- she should be warned about the possible ineffectiveness of contraceptives;
- she must confirm that she understands the precautions;
- she must understand the need and continuously use effective methods of contraception for one month before treatment with Roaccutane, during treatment and for a month after its completion (see section “Interaction with other drugs”); it is advisable to use 2 different methods of contraception at the same time, including barrier;
- she must have received a negative result from a reliable pregnancy test within 11 days before starting the drug; A pregnancy test is strongly recommended monthly during treatment and 5 weeks after the end of therapy;
- she should start treatment with Roaccutane only on the 2-3 day of the next normal menstrual cycle;
- she must understand the need for mandatory visits to the doctor every month;
- when treated for relapse of the disease, she must constantly use the same effective methods of contraception for one month before starting treatment with Roaccutane, during treatment and for a month after its completion, and also undergo the same reliable pregnancy test;
- she must fully understand the need for precautions and confirm her understanding and desire to use reliable methods of contraception as explained to her by the doctor.
Use of contraception as directed above during treatment with isotretinoin should be recommended even in women who do not routinely use contraception due to infertility (except in patients who have had a hysterectomy), amenorrhea, or who report not being sexually active.
The doctor must be sure that:
- the patient suffers from a severe form of acne (nodulocystic, conglobate acne or acne with a risk of scarring); acne that does not respond to other types of therapy;
- a negative result from a reliable pregnancy test was obtained before starting the drug, during therapy and 5 weeks after the end of therapy; the dates and results of the pregnancy test must be documented;
- the patient uses at least 1, preferably 2 effective methods of contraception, including a barrier method, for one month before starting treatment with Roaccutane, during treatment and for a month after its completion;
- the patient is able to understand and fulfill all of the above requirements for pregnancy protection;
- the patient meets all of the above conditions.
Pregnancy test
According to current practice, a pregnancy test with a minimum sensitivity of 25 mIU/ml should be performed in the first 3 days of the menstrual cycle:
Before starting therapy
To rule out possible pregnancy, the result and date of the initial pregnancy test must be recorded by a doctor before starting contraception. In patients with irregular menstruation, the timing of a pregnancy test depends on sexual activity and should be performed 3 weeks after unprotected intercourse. The doctor should inform the patient about contraceptive methods.
A pregnancy test is carried out on the day of Roaccutane's prescription or 3 days before the patient's visit to the doctor. The specialist should record the test results. The drug can only be prescribed to patients receiving effective contraception for at least 1 month before starting Roaccutane therapy.
During therapy
The patient must visit the doctor every 28 days. The need for monthly pregnancy testing is determined in accordance with local practice and taking into account sexual activity and previous menstrual irregularities. If indicated, a pregnancy test is performed on the day of the visit or three days before the visit to the doctor, the test results must be recorded.
End of therapy
5 weeks after the end of therapy, a test is performed to exclude pregnancy.
A prescription for Roaccutane for a woman capable of childbearing can be issued only for 30 days of treatment; continuation of therapy requires a new prescription of the drug by a doctor. It is recommended that a pregnancy test, writing a prescription and receiving the drug be carried out on the same day.
Roaccutane should be dispensed at a pharmacy only within 7 days from the date of issuing the prescription.
To help doctors, pharmacists and patients avoid the risk of fetal exposure to Roaccutane, the company aims to warn about the drug's teratogenicity and emphasize the absolute mandatory use of reliable contraceptive measures for women of childbearing age. The program contains the following materials:
for doctors:
- a doctor's guide to prescribing Roaccutane to women;
- informed consent form for the patient;
- form for recording drug prescriptions for women.
for the patient:
- patient information leaflet;
- what you need to know about contraception.
for the pharmacist:
- A pharmacist's guide to dispensing Roaccutane.
Full information about teratogenic risk and strict adherence to measures to prevent pregnancy should be provided to both men and women.
For male patients
Existing data suggest that in women, exposure to the drug from the semen and seminal fluid of men taking Roaccutane is not sufficient to cause the teratogenic effects of Roaccutane.
Men should exclude the possibility of taking the drug by other persons, especially women.
If, despite the precautions taken, pregnancy does occur during treatment with Roaccutane or within a month after its end, there is a high risk of very severe fetal malformations (in particular, from the central nervous system, heart and large blood vessels). In addition, the risk of spontaneous miscarriage increases.
If pregnancy occurs, Roaccutane therapy is discontinued. The advisability of maintaining it should be discussed with a doctor specializing in teratology.
Severe congenital malformations of the fetus in humans associated with the use of Roaccutane have been documented, including hydrocephalus, microcephaly, cerebellar malformations, anomalies of the external ear (microtia, narrowing or absence of the external auditory canal), microphthalmia, cardiovascular anomalies (tetralogy of Fallot, transposition of the great vessels, septal defects), malformations of the face (cleft palate), thymus gland, pathology of the parathyroid glands.
Because isotretinoin is highly lipophilic, it is very likely that it passes into breast milk. Due to possible side effects, Roaccutane should not be prescribed to nursing mothers.
Use for liver dysfunction
Contraindication: liver failure.
Use for renal impairment
In patients with severe renal impairment, treatment should be started with a lower dose (eg, 10 mg/day) and further increased to 1 mg/kg/day or the maximum tolerated.
Use in children
Contraindication: children under 12 years of age.
special instructions
Roaccutane should only be prescribed by physicians, preferably dermatologists, who are experienced in the use of systemic retinoids and are aware of the drug's teratogenicity risk. Both female and male patients should be given a copy of the Patient Information Leaflet.
To avoid accidental exposure of the drug to the body of other people, donated blood should not be taken from patients who are receiving or have recently (1 month) received Roaccutane.
It is recommended to monitor liver function and liver enzymes before treatment, 1 month after treatment, and then every 3 months or as indicated. A transient and reversible increase in liver transaminases was noted, in most cases within normal values. If the level of liver transaminases exceeds the norm, it is necessary to reduce the dose of the drug or discontinue it.
Fasting serum lipid levels should also be determined before treatment, 1 month after initiation, and then every 3 months or as indicated. Typically, lipid concentrations normalize after dose reduction or discontinuation of the drug, as well as with diet. It is necessary to monitor a clinically significant increase in triglyceride levels, since their rise above 800 mg/dL or 9 mmol/L can be accompanied by the development of acute pancreatitis, possibly fatal. In case of persistent hypertriglyceridemia or symptoms of pancreatitis, Roaccutane should be discontinued.
In rare cases, depression, psychotic symptoms, and very rarely, suicide attempts have been described in patients treated with Roaccutane. Although their causal relationship with the use of the drug has not been established, special caution should be exercised in patients with a history of depression and all patients should be monitored for the occurrence of depression during treatment with the drug, if necessary, referring them to an appropriate specialist. However, discontinuation of Roaccutane may not lead to the disappearance of symptoms and further observation and treatment by a specialist may be required.
In rare cases, at the beginning of therapy, an exacerbation of acne is observed, which resolves within 7-10 days without adjusting the dose of the drug.
Several years after the use of Roaccutane for the treatment of dyskeratosis, at a total course dose and duration of therapy higher than those recommended for the treatment of acne, bone changes developed, including premature closure of the epiphyseal growth plates, hyperostosis, calcification of ligaments and tendons. Therefore, when prescribing the drug to any patient, the ratio of possible benefits and risks should first be carefully assessed.
Patients receiving Roaccutane are recommended to use moisturizing ointment or body cream, lip balm to reduce dry skin and mucous membranes at the beginning of therapy.
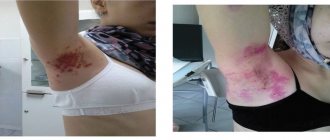
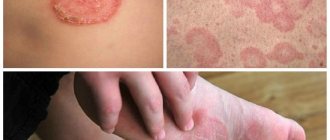
During post-marketing surveillance with the use of the drug Roaccutane, cases of severe skin reactions, such as erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been described. These events can be serious and can lead to disability, life-threatening conditions, hospitalization or death. Patients receiving Roaccutane require careful monitoring to identify severe skin reactions and, if necessary, consider discontinuing the drug.
While taking Roaccutane, pain in muscles and joints and an increase in serum creatinine phosphokinase are possible, which may be accompanied by a decrease in tolerance to intense physical activity.
Deep chemical dermoabrasion and laser treatment should be avoided in patients receiving Roaccutane, as well as for 5-6 months after the end of treatment due to the possibility of increased scarring in atypical places and the occurrence of hyper- and hypopigmentation. During treatment with Roaccutane and for 6 months after it, hair removal using wax applications cannot be performed due to the risk of epidermal detachment, scar development and dermatitis.
Since some patients may experience a decrease in night vision acuity, which sometimes persists even after the end of therapy, patients should be informed about the possibility of this condition, advising them to exercise caution when driving at night. Visual acuity must be carefully monitored.
Dryness of the conjunctiva of the eyes, corneal opacities, deterioration of night vision and keratitis usually disappear after discontinuation of the drug. If the mucous membrane of the eyes is dry, you can use applications of a moisturizing eye ointment or an artificial tear preparation. Patients with dry conjunctiva should be monitored for possible development of keratitis. Patients with vision complaints should be referred to an ophthalmologist and consider the advisability of discontinuing Roaccutane. If you are intolerant to contact lenses, you should use glasses during therapy.
Exposure to sunlight and UV rays should be limited. If necessary, use sunscreen with a high protection factor of at least 15 SPF.
Rare cases of the development of benign intracranial hypertension (“pseudotumor cerebri”) have been described, incl. when used in combination with tetracyclines. In such patients, Roaccutane should be discontinued immediately.
During therapy with Roaccutane, inflammatory bowel disease may occur. In patients with severe hemorrhagic diarrhea, Roaccutane should be immediately discontinued.
Rare cases of anaphylactic reactions that occurred only after previous external use of retinoids have been described. Severe allergic reactions dictate the need to discontinue the drug and carefully monitor the patient.
Patients at high risk (with diabetes, obesity, chronic alcoholism or lipid metabolism disorders) may require more frequent laboratory monitoring of glucose and lipid levels when treated with Roaccutane.
If diabetes is present or suspected, more frequent monitoring of glycemia is recommended.