Diabetes mellitus is such a common disease that there is hardly a person who has not heard of it or does not know that diabetes is manifested by elevated levels of sugar (glucose) in the blood. But not everyone suspects how dangerous it is. The fact is that diabetes has been the leading cause of death in the world for many decades. And every year the situation not only does not improve, but only gets worse. So, if you look at the report of the World Health Organization, the numbers are simply terrifying: from 2000 to 2022, mortality from diabetes increased by 70% and in 2022 amounted to 1.5 million people. That is, this is more than the new coronavirus infection claimed in a year. Moreover, 85% of these were patients with type 2 diabetes mellitus. But many people take this disease as an almost “normal condition” that does not require special attention. In fact, it is long-term neglect of diabetes that leads to such sad consequences.
Can you “feel” type 2 diabetes?
Unfortunately, slightly elevated blood sugar levels are not noticeable. This is the insidiousness of type 2 diabetes - a person feels good, but he already develops complications of diabetes. With higher glucose levels, dry mouth, thirst, and increased urination (especially at night) . But these symptoms are only a manifestation of the body’s protective reaction to elevated blood sugar levels and do not at all indicate complications of diabetes. By urinating more frequently, the body tries to get rid of excess glucose. Therefore, with normalization of blood sugar, these symptoms disappear very quickly. Chronic complications of diabetes, or also called “late complications,” arise due to damage to blood vessels and nerve endings, which are “sugared” by excess amounts of glucose. The very first manifestations of complications are: blurred vision, numbness of the toes, a feeling of “pins and needles” in the legs, and in men - impaired potency . In a later period, hemorrhages in the retina may occur, which often result in complete loss of vision, strokes, heart attacks , as well as gangrene of the lower extremities , requiring amputation of the legs.
Use of Glidiab in complex therapy of type 2 diabetes mellitus
WITH
Diabetes mellitus is one of the most common endocrine diseases, representing a significant problem for national health services due to the development of vascular complications of diabetes, which cause early disability and high mortality.
Of the total number of diabetic patients, about 90% are patients suffering from type 2 diabetes mellitus
.
The main role in the mechanisms of pathogenesis of vascular complications of diabetes mellitus belongs to hyperglycemia, and in type 2 diabetes mellitus, lipid metabolism disorders.
The European Bureau of the International Federation of Diabetes and the WHO European Bureau in 1998 proposed criteria for metabolic compensation in patients with type 2 diabetes, which are presented in Table 1.
In type 2 diabetes mellitus, carbohydrate metabolism disorders are combined with pronounced changes in lipid metabolism. In this regard, when considering compensation of metabolic processes, one should take into account not only the glucose content in the blood plasma, but also indicators of lipid metabolism, which to one degree or another correlate with the risk of developing vascular complications of diabetes (Table 2).
The adequacy of diabetes therapy remains the most pressing issue, since it has been established that hyperglycemia is the trigger for many pathogenetic mechanisms that contribute to the development of vascular complications. Strict compensation for diabetes, i.e. By maintaining a normal (or close to normal) blood glucose concentration for a long time, it is possible to delay or delay the development of late complications of diabetes mellitus.
Treatment of type 2 diabetes mellitus is complex and includes diet, dosed physical activity, patient education and self-control of diabetes, drug therapy, prevention and treatment of late complications of diabetes mellitus.
For drug therapy of patients with type 2 diabetes, drugs with different mechanisms of action are used:
drugs that reduce the absorption of carbohydrates in the gastrointestinal tract (acarbose, etc.); biguanides (metformin); sulfonylurea drugs that stimulate insulin secretion, which include 2nd generation drugs: glibenclamide, glipizide, gliclazide (Glidiab), gliquidone and 3rd generation sulfonylurea drugs; short-acting drugs or prandial glucose regulators, which are derivatives of amino acids - repaglinide and nateglinide. In cases where it is not possible to achieve compensation for diabetes mellitus with the help of oral hypoglycemic drugs (in patients with type 2 diabetes mellitus with a pronounced defect of b-cells of the pancreatic islets), the use of combination therapy (oral hypoglycemic therapy + insulin therapy, more often with drugs of medium duration of action) is recommended at night or 2 times a day).
The purpose of the study was to assess the effectiveness, safety and tolerability of the drug Glidiab.
(pharmaceutical), registered with the Ministry of Health of the Russian Federation (P No. 011468/01–1999), its effect on the state of carbohydrate and lipid metabolism, lipid peroxidation, the state of the antioxidant system and insulin resistance in patients with type 2 diabetes mellitus. As a control or comparison group, we formed a group of patients suffering from type 2 diabetes mellitus, in whom the effect of diet therapy on the listed indicators was studied.
Material and methods
We examined 22 patients with type 2 diabetes mellitus (18 women and 4 men), average age 60.3 years, average disease duration 6.4 years. The duration of treatment with Glidiab was at least 12 weeks. The dose of the drug was selected individually depending on the patient’s weight and fasting and postprandial glucose levels during the initial examination. Dynamic monitoring of glycemic levels was carried out every 14 days, according to which, if necessary, therapeutic doses of the drug were adjusted. All observed patients had vascular complications of varying severity.
The second group of subjects included 30 patients (21 women and 9 men) with type 2 diabetes mellitus who were on diet therapy for 3 months. Age – from 42 to 70 years (average 58.77±8.86 years). The duration of the disease is from 1 month to 5 years. The absence of drug glucose-lowering therapy was additional evidence of the presence of a mild form of type 2 diabetes mellitus.
The effectiveness of the clinical use of the drug Glidiab was assessed by the dynamics of compensation of diabetes mellitus, for which the content of glycosylated hemoglobin was determined (before the start of insulin therapy and after 3 months of continuous treatment), by the level of glycemia on an empty stomach and two hours after meals, the frequency of cases of ketoacidosis and hypoglycemic conditions , as well as the content of immunoreactive insulin (IRI) and C-peptide in the blood serum of patients.
To establish the coefficient of insulin resistance, the level of immunoreactive insulin was determined in all patients. To assess the severity of hyperinsulinemia, a study of the amount of C-peptide was carried out. The severity of insulin resistance was determined by the index or coefficient of insulin resistance (IRI), determined
according to the formula = (fasting glycemia * IRI) / 25
In addition, the effect of Glidiab on the state of lipid metabolism was studied, for which the content of total cholesterol, triglycerides, low- and high-density lipoprotein cholesterol in the blood serum was determined (both before and after 3 months of Glidiab therapy). We judged the safety of Glidiab use by the content of creatinine, urea, liver transaminases (AST and ALT), potassium, and sodium in the blood serum, determined before and after the end of treatment.
The hemocoagulation potential of the blood was assessed using thromboelastography indicators (r, k, ma, t, S, C, E). In platelet-poor blood plasma, fibrinogen concentrations were determined using the C1au88 method, the content of soluble fibrin-monomer complexes (SFMC), fibrinolysis activity using the euglobulin lysis method, and the values of activated partial thromboplastin time (APTT), activated recalcification time (AVR), and thrombin time ( PTV) using a coagulometer (Schnitger und Gross, Germany). Kits (Moscow) were used in the work.
The effect of Glidiab on lipid peroxidation (LPO) and the state of the body's antioxidant system was assessed based on the levels of malondialdehyde, superoxide dismutase and glutathione peroxidase levels before and at the end of the observed period.
Tolerability of treatment with Glidiab produced by pharmaceutical manufacturers was assessed by the presence of the frequency of adverse events and adverse reactions. The safety of using this drug was determined by the frequency of episodes of hypoglycemia, the frequency of allergic reactions, as well as by biochemical and general blood and urine tests.
Results and discussion
Glidiab is a hypoglycemic drug from the group of second-generation sulfonylurea derivatives - gliclazide, which has been used for decades in the treatment of type 2 diabetes mellitus. Gliclazide (Glidiab) is derived from the combination of a sulfonylurea radical and a nitrogen-containing heterocyclic ring with an intra-ring bridge. This is a chemical substance that, in addition to its sugar-lowering effect, has a pronounced effect on hematological parameters, rheological properties of blood, the hemostatic system and microcirculation. Gliclazide (Glidiab) is completely absorbed from the gastrointestinal tract. Its maximum concentration in the blood is detected 2–6 hours after taking the drug, its half-life in circulation is 6–15 hours, and the duration of the effect is 10–15 hours. The therapeutic dose of gliclazide (Glidiab) is 40–320 mg/day. Glidiaba tablet contains 80 mg of gliclazide. Metabolism of gliclazide occurs in the liver with the formation of 7–8 metabolites that do not have hypoglycemic activity. The main metabolite of gliclazide (Glidiab), a carboxylic acid derivative, has an inhibitory effect on platelet aggregation and, apparently, on other vascular effects of gliclazide. About 65% of gliclazide (Glidiab) is excreted in the form of metabolites in the urine and 12–20% through the gastrointestinal tract. The main effect of Glidiab on the state of carbohydrate metabolism, like other representatives of sulfonylurea drugs, is due to the stimulation of insulin secretion by the islet apparatus of the pancreas.
Gliclazide (Glidiab) is known to restore the first peak of insulin secretion in patients with type 2 diabetes, promoting more physiological insulin secretion. In addition, Glidiab increases the sensitivity of peripheral tissues to insulin. There is evidence that the drug stimulates the activity of intracellular enzymes (including muscle glycogen synthetase).
By restoring the early peak of insulin secretion, Glidiab compares favorably with other sulfonylurea drugs, such as glibenclamide and chlorpropamide, which act mainly on phase 2 of insulin secretion.
This pharmacological effect of the drug is very important, since it does not lead to a sharp increase in body weight and does not cause such pronounced hyperinsulinemia. Gliclazide (Glidiab) also reduces postprandial glycemic levels to a certain extent. In addition, as noted above, Glidiab reduces platelet adhesion and aggregation, delays the development of parietal thrombosis, and increases vascular fibrinolytic activity. The drug normalizes vascular permeability and prevents the development of microthrombosis (DIC syndrome), which naturally affects the reduction in the rate of development of atherosclerosis, leading to macroangiopathies. The above-described properties of Glidiab cause a decrease in the severity of vascular disorders (micro- and macroangiopathies) in type 2 diabetes mellitus.
State of carbohydrate metabolism
.
Indicators of the state of carbohydrate metabolism in the examined patients are presented in Table 3. In patients undergoing Glidiab therapy, fasting glycemia remained virtually unchanged during the treatment period and remained within the limits of those indicators that indicate the state of compensation.
The levels of glycosylated hemoglobin in the blood also remained virtually unchanged during the treatment period, and a slight decrease in glycohemoglobin at the end of treatment was only 1.3%. In patients with type 2 diabetes mellitus undergoing diet therapy, there was a significant decrease in fasting glycemia from 7.40±1.11 mmol/l to 6.69±1.11 (p=0.01) with a moderate decrease in the level of glycosylated hemoglobin in the blood from 6.88±0.51 to 6.69±0.77% (p=0.34), i.e. by 2.6%, which is statistically unreliable.
The state of compensation of carbohydrate metabolism in patients with type 2 diabetes mellitus during therapy with Glidiab or diet was accompanied by an improvement in the indicators of b-cell function of the pancreatic islets, which are presented in Table 4.
Improvement in indicators of the state of carbohydrate metabolism in the examined patients undergoing Glidiab therapy was accompanied by an increase in the content of IRI in the blood serum from 17.06±8.75 to 20.16±9.25 µU/ml (p<0.05) with almost unchanged level of C-peptide in blood serum (from 3.14±0.99 to 3.02±1.13 ng/ml; p=0.93).
In patients suffering from type 2 diabetes mellitus and on a diet, there was a slight increase in the level of C-peptide in the blood serum (from 2.61±1.48 to 2.72±0.94 ng/ml; p=0.92) with a statistically significant decrease in the content of IRI in the blood serum (17.3±9.46 to 14.34±6.30 µU/ml; p=0.01), which is also evidence of an increase in the sensitivity of peripheral tissues to insulin.
The data obtained show that maintaining compensation of carbohydrate metabolism in patients with type 2 diabetes mellitus who are on therapy with Glidiab and diet is carried out by mechanisms that, although slightly, differ from each other.
Thus, if compensation of carbohydrate metabolism is achieved under the influence of previous oral hypoglycemic therapy, further maintenance of carbohydrate metabolism close to normal values recommended by WHO and the International Diabetes Federation is achieved through further stimulation of insulin secretion with a moderate decrease in the content of C-peptide in the blood serum.
It is known that several mechanisms are involved in the pathogenesis of type 2 diabetes mellitus, the main of which are: insulin resistance and insufficiency of insulin secretion by b-cells of the islet apparatus of the pancreas. The role of insulin resistance in the body is not limited to its effect on carbohydrate metabolism.
Insulin resistance is also combined with other pathological conditions (hypertension, atherosclerosis and macroangiopathy, polycystic ovary syndrome, etc.), which can be both independent diseases and “complications, and possibly combined conditions” with type 2 diabetes mellitus. At the same time, insulin resistance is also involved in the development of vascular complications of diabetes mellitus, which are still the main cause of early disability and high mortality observed in this disease. In light of the above, the keen interest of doctors in the problem of insulin resistance in diabetes mellitus and the possibility of actively influencing the degree of its severity becomes understandable.
Determination of the insulin resistance index in the patients we examined showed that during Glidiab therapy the latter remained virtually unchanged. Before starting therapy with Glidiab, the insulin resistance index was 4.83±0.35, and after 3 months of therapy – 5.49±1.04, which, in our opinion, reflects the state of maintaining glucose homeostasis in patients with type 2 diabetes mellitus, depending on the condition the initial compensation of carbohydrate metabolism and the mechanisms by which a state of carbohydrate metabolism close to normal can be maintained for a long time.
In patients receiving Glidiab therapy, the state of lipid metabolism was also studied (Table 5).
As can be seen from the data presented in Table 5, the content of triglycerides in the blood serum practically did not change, the content of low-density lipoproteins decreased from 3.38 ± 0.61 to 3.01 ± 0.57 mmol/l (by 11%) and total cholesterol from 5.24±0.93 to 5.19±0.95 mmol (by 1%) with a simultaneous moderate increase in the level of high-density lipoproteins - from 1.32±0.21 to 1.37±0.57 mmol/ l (+3.7%). However, the observed change in lipid content in the blood serum was statistically insignificant.
In patients with type 2 diabetes mellitus who were on diet therapy, changes in lipid levels were also noted (Table 6).
The data presented in Table 6 indicate that diet therapy, like Glidiab therapy, promotes a moderate decrease in the content of total cholesterol, low-density lipoproteins, and triglycerides with a slight increase in the level of high-density lipoprotein cholesterol. Changes in the content of lipid metabolism indices in patients undergoing diet therapy are also statistically unreliable.
In the patients we examined during treatment with Glidiab, a moderate decrease in body mass index was noted from 32.1 to 31.95. Naturally, weight loss was facilitated not only by Glidiab therapy, but to a greater extent by following doctors’ advice to follow the recommended diet. A decrease in body mass index from 29.86±4.15 to 29.02±3.88 was also observed in patients on diet therapy.
Recent studies have shown that the state of lipid peroxidation (LPO) and the antioxidant defense system are of great importance in the pathogenesis of vascular changes. Data on the state of lipid peroxidation and the activity of antioxidant enzymes during treatment with Glidiab are presented in Table 7.
The content of lipid peroxidation products and the activity of antioxidant enzymes in patients on diet are shown in Table 8. The level of lipid peroxidation products (malondialdehyde and diene conjugates) decreased during both types of therapy, and this decrease was even more pronounced during diet therapy. As for the activity indicators of antioxidant enzymes and, in particular, the main one, superoxide dismutase, an increase in activity was observed during Glidiab therapy, while a decrease was observed during diet therapy. At first glance this may seem paradoxical. However, the fact of an increase in the activity of antioxidant defense enzymes during Glidiab therapy may indicate an improvement in the overall balance between LPO indicators and the activity of antioxidant defense enzymes. However, given that the content of LPO products during diet therapy turned out to be more pronounced than during treatment with Glidiab, we must remember that the overall balance of the equilibrium of these two systems (LPO and the activity of antioxidant enzymes) in both types of therapy is almost the same.
The study showed that the pharmaceutical drug Glidiab (gliclazide) has a pronounced hypoglycemic activity
, which is accompanied by maintaining the level of glycemia within the limits almost allowed for patients with type 2 diabetes mellitus, as well as an increase in the content of IRI in the blood serum, which is evidence of its stimulating effect on insulin secretion in the b-cells of the pancreatic islets.
Diet therapy, like treatment with Glidiab, is accompanied by the preservation of the degree of compensation for the state of carbohydrate metabolism achieved by previous glucose-lowering therapy due to significant stimulation of insulin secretion by b-cells of the pancreatic islets.
In addition to a pronounced hypoglycemic effect, Glidiab also has a positive effect on the state of lipid metabolism, which is accompanied by a moderate decrease in the content of total cholesterol, triglycerides, low-density lipoprotein cholesterol and an increase in high-density lipoprotein cholesterol. Similar changes in lipid metabolism, but to a lesser extent, are also present in patients on diet therapy.
The insulin resistance index in patients on diet therapy decreased from 5.12 to 3.83, while during Glidiab therapy there was a moderate increase due to the stimulating effect of Glidiab on insulin secretion, which is probably a kind of compensatory reaction of the body to maintain glucose homeostasis .
A decrease in lipid peroxidation products is more pronounced in patients on diet therapy. However, in patients receiving Glidiab therapy, there was a greater increase in the activity of enzymes of the antioxidant system.
Not a single patient experienced any side effects or adverse events throughout the entire course of therapy. All patients tolerated therapeutic doses of the drug well (80 mg once or twice a day). Indicators of a general blood test, urine, biochemical blood parameters, including liver tests and indicators of kidney function (creatinine and urea content), remained within normal values.
Thus, the drug Glidiab meets all the requirements for drugs used to treat type 2 diabetes mellitus.
Causes of type 2 diabetes mellitus
First of all, blood sugar levels are affected by what we eat and at what time, but especially in what quantity. Therefore, poor nutrition, of course, is one of the main causes of diabetes. This means... by correcting it, you can significantly reduce the risk of diabetes, and in some cases even prevent its development.
But, before we begin to describe the principles of nutrition, we need to figure out what other factors provoke the appearance of type 2 diabetes in order to understand who is at increased risk and who needs to pay special attention to their eating habits.
Risk factors for type 2 diabetes mellitus are:
- presence of close relatives with diabetes;
- overweight (BMI* from 25 to 29.9) or obesity (BMI more than 30). However, if you have an ideal body weight, but have a large waist (more than 80 cm in women, and more than 94 cm in men), this is also a risk factor for diabetes;
- increased glucose levels during pregnancy (gestational diabetes);
- elderly age;
- lack of physical activity;
- smoking.
*In medicine, the body mass index (BMI) is used to assess obesity, which is calculated using the formula: weight (kg)/height (m2).
Unfortunately, we cannot influence such risk factors as heredity and age, but other risks can be significantly reduced. And first of all, this applies to nutrition!
Ongliza 5mg n30 film-coated tablets
Latin name
Ongliza
Release form
Pills
Package
30 pcs
pharmachologic effect
Onglyza - saxagliptin - is a potent selective reversible competitive inhibitor of dipeptidyl peptidase-4 (DPP-4).
In patients with type 2 diabetes mellitus, taking saxagliptin leads to suppression of the activity of the DPP-4 enzyme within 24 hours. After oral glucose administration, inhibition of DPP-4 leads to a 2-3 fold increase in the concentration of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), a decrease in the concentration of glucagon and an increase in the glucose-dependent response of beta cells, which leads to an increase in the concentration of insulin and C-peptide.
Release of insulin from pancreatic beta cells and decreased release of glucagon from pancreatic alpha cells results in decreased fasting and postprandial glycemia.
The effectiveness and safety of saxagliptin when taken in doses of 2.5 mg, 5 mg and 10 mg once a day were studied in six double-blind, placebo-controlled studies involving 4148 patients with type 2 diabetes mellitus. Taking the drug was accompanied by a statistically significant improvement in glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) compared to the control.
Patients in whom the target glycemic level could not be achieved while taking saxagliptin as monotherapy were additionally prescribed metformin, glibenclamide or thiazolidinediones. When taking saxagliptin at a dose of 5 mg, a decrease in HbA1c was noted after 4 weeks and FPG after 2 weeks. In the group of patients receiving saxagliptin in combination with metformin, glibenclamide or thiazolidinediones, a decrease in HbA1c was also observed after 4 weeks and FPG after 2 weeks.
The effect of saxagliptin on the lipid profile was similar to that of placebo. No weight gain was observed during saxagliptin therapy.
Indications
Type 2 diabetes mellitus in addition to diet and exercise to improve glycemic control as:
— monotherapy;
— initial combination therapy with metformin;
- addition to monotherapy with metformin, thiazolidinediones, sulfonylurea derivatives, in the absence of adequate glycemic control on this therapy.
Contraindications
- type 1 diabetes mellitus (use not studied);
- use in combination with insulin (not studied);
- diabetic ketoacidosis;
- congenital galactose intolerance, lactase deficiency and glucose-galactose malabsorption;
- pregnancy;
- lactation;
- age under 18 years (safety and effectiveness have not been studied);
- increased individual sensitivity to any component of the drug.
Use during pregnancy and breastfeeding
Due to the fact that the use of saxagliptin during pregnancy has not been studied, the drug should not be prescribed during this period.
It is not known whether saxagliptin passes into breast milk. Due to the fact that the possibility of saxagliptin passing into breast milk cannot be excluded, breastfeeding should be stopped during treatment with saxagliptin or therapy should be discontinued, taking into account the balance of risk for the child and benefit for the mother.
special instructions
The use of Ongliza® in combination with insulin, as well as as part of triple therapy with metformin and thiazolidinediones or metformin and sulfonylurea derivatives, has not been studied.
Patients with impaired renal function.
Dose adjustment is recommended for patients with moderate to severe renal impairment, as well as for patients on hemodialysis. Before starting therapy and periodically during treatment with the drug, it is recommended to evaluate renal function.
Use in combination with drugs that can cause hypoglycemia.
Sulfonylureas may cause hypoglycemia; therefore, a reduction in the dose of sulfonylureas may be necessary to reduce the risk of hypoglycemia when used concomitantly with Onglyza.
Hypersensitivity reactions.
The drug should not be prescribed to patients who have had serious hypersensitivity reactions when using other DPP-4 inhibitors.
Elderly patients.
According to clinical studies, efficacy and safety rates in patients aged 65 years and older did not differ from those in younger patients. However, increased individual sensitivity to saxagliptin in some elderly patients cannot be excluded.
Saxagliptin and its main metabolite are partially eliminated by the kidneys, so it must be taken into account that elderly patients are more likely to have decreased renal function. Ongliza® contains lactose. Patients with congenital galactose intolerance, lactase deficiency and glucose-galactose malabsorption should not take this drug.
Influence on the ability to drive vehicles and operate machinery.
No studies have been conducted to study the effect of saxagliptin on the ability to drive vehicles and operate machinery. Please note that saxagliptin may cause dizziness.
Compound
active substance: saxagliptin 5 mg.
excipients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, hydrochloric acid 1 M or sodium hydroxide solution 1 M, Opadry II white (polyvinyl alcohol, titanium dioxide, macrogol (PEG 3350), talc), Opadry II yellow (alcohol polyvinyl, titanium dioxide, macrogol (PEG 3350), talc, yellow iron oxide dye (E172), Opacode blue ink (shellac in ethyl alcohol, FD&C Blue #2/indigo carmine aluminum pigment (E132), n-butyl alcohol, propylene glycol, isopropyl alcohol, 28% ammonium hydroxide).
Directions for use and doses
The drug is prescribed orally, regardless of food intake.
For monotherapy, the recommended dose of saxagliptin is 5 mg 1 time / day.
In combination therapy, the recommended dose of saxagliptin is 5 mg 1 time / day in combination with metformin, thiazolidinediones or sulfonylurea derivatives.
When starting combination therapy with metformin, the recommended dose of saxagliptin is 5 mg 1 time / day, the initial dose of metformin is 500 mg / day. In case of inadequate response, the dose of metformin may be increased.
If you miss taking Ongliza®, the missed tablet should be taken as soon as the patient remembers, but you should not take a double dose of the drug within one day.
For patients with mild renal failure (creatinine clearance >50 ml/min), no dose adjustment is required. For patients with moderate or severe renal impairment (CR
For mild, moderate and severe liver dysfunction, no dose adjustment is required.
No dose adjustment is required in elderly patients. However, when choosing a dose, it should be taken into account that in this category of patients a decrease in renal function is more likely.
The safety and effectiveness of the drug in patients under 18 years of age have not been studied.
When used concomitantly with strong CYP 3A4/5 inhibitors, such as ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir and telithromycin, the recommended dose of Onglyza® is 2.5 mg 1 time / day
Drug interactions
Analysis of data from clinical studies suggests that the risk of clinically significant interactions between saxagliptin and other drugs when used together is low.
The metabolism of saxagliptin is predominantly mediated by the cytochrome P450 3A4/5 isoenzyme system (CYP3A4/5). In vitro studies have shown that saxagliptin and its main metabolite do not inhibit CYP isoenzymes 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1 and 3A4 and do not induce CYP isoenzymes 1A2, 2B6, 2C9, and 3A4. In studies involving healthy volunteers, the pharmacokinetic parameters of saxagliptin and its main metabolite were not significantly changed by metformin, glibenclamide, pioglitazone, digoxin, simvastatin, diltiazem, ketoconazole, omeprazole, a combination of aluminum hydroxide, magnesium hydroxide and simethicone, as well as famotidine. Saxagliptin does not significantly change the pharmacokinetic parameters of metformin, glibenclamide, pioglitazone, digoxin, simvastatin, diltiazem or ketoconazole.
The effect of inducers of CYP 3A4/5 isoenzymes on the pharmacokinetics of saxagliptin has not been studied. However, the combined use of saxagliptin and inducers of CYP 3A4/5 isoenzymes, such as carbamazepine, dexamethasone, phenobarbital, phenytoin and rifampicin, may lead to a decrease in the concentration of saxagliptin in plasma and an increase in the concentration of its main metabolite. The effects of smoking, dietary intake, herbal preparations and alcohol consumption on saxagliptin therapy have not been studied.
Overdose
Symptoms of intoxication are not described with long-term use of the drug in doses up to 80 times higher than recommended.
Treatment: in case of overdose, symptomatic therapy should be used. Saxagliptin and its main metabolite are eliminated from the body by hemodialysis (removal rate: 23% of the dose in 4 hours).
Storage conditions
At a temperature not exceeding 30°C
Best before date
3 years
Conditions for dispensing from pharmacies
On prescription
How can dietary changes help prevent diabetes?
When it comes to preventing diabetes, many people think about the need to almost completely limit carbohydrates in their diet. And at first glance, this looks quite logical - after all, it is carbohydrates that increase blood sugar levels. But still there is no need to rush into this. Firstly, carbohydrates are different, and secondly, they perform many useful functions in our body. You just need to understand what carbohydrates you can afford and at what time.
Carbohydrates differ from each other in the rate of absorption, that is, in the time they enter the blood. Therefore, they can be divided into “fast”, “medium” and “long” carbohydrates. “Fast” include foods and drinks to which sugar is added, including products that contain “natural sugars” (fructose) - honey, fruit juices, concentrates. They appear in the blood literally a few minutes after their consumption, sharply increasing blood sugar levels. These carbohydrates have the highest glycemic index*. “Medium” include : bread, cereals, pasta, potatoes, beets, carrots, corn. This kind of carbohydrates is absorbed much more slowly, and accordingly, glucose in the blood will also increase more slowly. But, if they are subjected to prolonged cooking, their absorption will be significantly accelerated. “Long” carbohydrates contain a large amount of plant fiber: tomatoes, cucumbers, zucchini, eggplant, cabbage, lettuce, and, of course, greens. And they are the most difficult for the body to process, so the sugar level will rise very slowly and evenly. Long carbohydrates have the lowest glycemic index.
*The glycemic index is a relative indicator that reflects the rate at which blood sugar levels rise after digesting certain foods.
Ongliza 5 mg 30 pcs. film-coated tablets
pharmachologic effect
Hypoglycemic agent.
Composition and release form Ongliza 5 mg 30 pcs. film-coated tablets
Tablets - 1 tablet:
- active ingredients: saxagliptin 2.5 mg or 5.0 mg in the form of saxagliptin hydrochloride;
- excipients: lactose monohydrate 99.0 mg, microcrystalline cellulose 90.0 mg, croscarmellose sodium 10.0 mg, magnesium stearate 1.0 mg, 1 M hydrochloric acid solution - required amount, Opadry II white (% w/w) 26 .0 mg [polyvinyl alcohol 40%, titanium dioxide 25%, macrogol (PEG 3350) 20.2%, talc 14.8%], Opadry II yellow (% w/w) 7.0 mg [polyvinyl alcohol 40%, titanium dioxide 24.25%, macrogol (PEG 3350) 20.2%, talc 14.8%, yellow iron oxide dye (E172) 0.75%] (for a dosage of 2.5 mg), Opadry II pink (% weight /weight) 7.0 mg [polyvinyl alcohol 40%, titanium dioxide 24.25%, macrogol (PEG 3350) 20.2%, talc 14.8%, dye red iron oxide (E172) 0.75%] (for dosage 5.0 mg), Opacode blue ink (% w/w) [shellac 45% in ethyl alcohol 55.4%, FD&C Blue #2 / indigo carmine aluminum pigment (E132) 16%, n-butyl alcohol 15%, propylene glycol 10.5%, isopropyl alcohol 3%, 28% ammonium hydroxide 0.1%.] - required amount.
Very small amounts of shellac and FD&C Blue #2/indigo carmine aluminum pigment remain on the tablets when marking is applied. The solvents contained in the ink are removed during the manufacturing process.
10 tablets in an aluminum foil blister; 3 blisters with instructions for use in a cardboard box with first opening control. The blister has a perforation, dividing it into 10 rectangular zones according to the number of tablets.
Description of the dosage form
Film-coated tablets, 2.5 mg: round, biconvex, pale yellow to light yellow film-coated tablets, marked “2.5” on one side and “4214” on the other side, printed in blue. Film-coated tablets, 5 mg: round, biconvex, pink film-coated tablets, marked “5” on one side and “4215” on the other side, printed in blue.
Directions for use and doses
Inside, regardless of food intake. The tablets should be swallowed whole, without chewing, crushing or breaking.
Monotherapy: the recommended dose of saxagliptin is 5 mg once daily.
Combination therapy: the recommended dose of saxagliptin is 5 mg 1 time per day in combination with metformin, thiazolidinediones, sulfonylurea derivatives, insulin (including in combination with metformin); When added to a combination of metformin and sulfonylurea derivatives, the recommended dose of saxagliptin is 5 mg once daily.
When starting combination therapy with metformin, the recommended dose of saxagliptin is 5 mg once a day, the initial dose of metformin is 500 mg per day. In case of inadequate response, the dose of metformin may be increased. If you miss taking Ongliza®, the missed tablet should be taken as soon as the patient remembers, but you should not take a double dose of the drug within one day.
Use in special patient groups
Patients with impaired renal function
For patients with mild renal failure (creatinine clearance >50 ml/min), no dose adjustment is required. For patients with moderate or severe renal impairment (CR
The use of saxagliptin in patients on peritoneal dialysis has not been studied. It is recommended that renal function be assessed before initiating saxagliptin therapy and during treatment.
Patients with liver dysfunction
For mild, moderate and severe liver dysfunction, no dose adjustment is required.
Elderly patients
No dose adjustment is required in elderly patients. However, when choosing a dose, it should be taken into account that in this category of patients a decrease in renal function is more likely.
Children
The safety and effectiveness of the drug in patients under 18 years of age have not been studied.
Concomitant use with strong CYP3A4/5 inhibitors
When used concomitantly with strong CYP3A4/5 inhibitors, such as ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir and telithromycin, the recommended dose of Onglyza® is 2.5 mg once daily.
Pharmacodynamics
Saxagliptin is a potent selective reversible competitive inhibitor of dipeptidyl peptidase-4 (DPP-4). In patients with type 2 diabetes mellitus (T2DM), taking saxagliptin leads to suppression of the activity of the DPP-4 enzyme within 24 hours. After oral glucose administration, inhibition of DPP-4 leads to a 2-3 fold increase in the concentration of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), a decrease in the concentration of glucagon and an increase in the glucose-dependent response of beta cells, which leads to an increase in the concentration of insulin and C-peptide. Release of insulin from pancreatic beta cells and decreased release of glucagon from pancreatic alpha cells results in decreased fasting and postprandial glycemia.
Clinical efficacy and safety
In double-blind, randomized, controlled clinical trials, more than 17,000 patients with T2DM were treated with saxagliptin.
Glycemic control
The efficacy and safety of saxagliptin at doses of 2.5 mg, 5 mg and 10 mg once daily were studied in six double-blind, placebo-controlled studies involving 4148 patients with T2DM. Taking the drug was accompanied by a statistically significant improvement in glycosylated hemoglobin (HbAlc), fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) compared to the control.
Saxagliptin was prescribed as monotherapy or combination therapy. Combination therapy with saxagliptin was prescribed additionally to patients uncompensated by monotherapy with metformin, glibenclamide, thiazolidinediones or insulin, or as a starting combination with metformin to patients uncompensated by diet and exercise. When taking saxagliptin at a dose of 5 mg, a decrease in HbAlc was noted after 4 weeks and FPG after 2 weeks.
In the group of patients receiving saxagliptin in combination with metformin, glibenclamide or thiazolidinediones, a decrease in HbAlc was also observed after 4 weeks and FPG after 2 weeks.
A study of combination therapy with saxagliptin and insulin (including in combination with metformin) involving 455 patients with T2DM demonstrated a significant reduction in HbAlc and PPG compared with placebo.
A study of saxagliptin in combination with metformin and sulfonylureas in 257 patients with T2DM demonstrated a significant reduction in HbAlc and PPG compared to placebo in combination with metformin and sulfonylureas. The effect of saxagliptin on the lipid profile was similar to that of placebo. No weight gain was observed during saxagliptin therapy. In a head-to-head study of 858 patients with T2DM, adding Onglyza 5 mg to metformin compared with glipizide to metformin demonstrated comparable reductions in HbAlc but was associated with significantly fewer episodes of hypoglycemia - 3% of cases compared to 36.3%. with the addition of glipizide, and no weight gain in patients receiving saxagliptin therapy (-1.1 kg from baseline in the saxagliptin group, +1.1 kg in the glipizide group). By week 104 of therapy, at least one episode of hypoglycemia occurred in 3.5% of patients in the saxagliptin and metformin group, and in 38.4% in the glipizide and metformin group; the change in body weight from baseline was -1.5 kg and +1.3 kg, respectively.
Cardiovascular outcomes
The SAVOR (Evaluation of Cardiovascular Outcomes in Patients with Diabetes Taking Saxagliptin) study examined cardiovascular outcomes in 16,492 patients with T2DM (12,959 patients with documented cardiovascular disease (CVD), 3,533 patients with multiple cardiovascular risk factors) and values 6.5%
Overall mortality was comparable between the saxagliptin and placebo groups (HR 1.11; 95% CI 0.96, 1.27).
The study noted an increase in hospitalization for chronic heart failure in the saxagliptin group (3.5%, 289 patients) compared with placebo (2.8%, 228 patients) with nominal statistical significance (i.e., not adjusted for multiple factors). endpoints) (RR 1.27; 95% CI 1.07, 1.51; P=0.007). Patients with chronic heart failure or renal failure who received saxagliptin did not experience a higher incidence of the primary endpoint, secondary endpoint, or all-cause mortality compared with the placebo group. In the saxagliptin group, the dynamics of HbAlc values were significantly more pronounced, and the percentage of patients who achieved the target HbAlc value was higher than in the placebo group. At the same time, significantly fewer patients required intensification of hypoglycemic therapy or the addition of insulin in the saxagliptin group than in the placebo group.
Pharmacokinetics
In patients with T2DM and healthy volunteers, similar parameters of the pharmacokinetics of saxagliptin and its main metabolite were noted. Saxagliptin is rapidly absorbed after oral administration on an empty stomach, with maximum plasma concentrations of saxagliptin and the major metabolite (Cmax) achieved within 2 hours and 4 hours, respectively. With an increase in the dose of saxagliptin, a proportional increase in Cmax and the area under the concentration-time curve (AUC) of saxagliptin and its main metabolite was noted. Following a single 5 mg oral dose of saxagliptin to healthy volunteers, the mean AUC values of saxagliptin and its major metabolite were 78 ngch/ml and 214 ngh/ml, and plasma Cmax values were 24 ng/ml and 47 ng/ml, respectively.
The average terminal half-life (t1/2) of saxagliptin and its main metabolite was 2.5 hours and 3.1 hours, respectively, and the average t1/2 of plasma DPP-4 inhibition was 27 hours. Inhibition of DPP-4 activity in plasma in for at least 24 hours after administration of saxagliptin is due to its high affinity for DPP-4 and prolonged binding to it. No noticeable accumulation of saxagliptin and its main metabolite was observed during long-term administration of the drug once a day. There was no dependence of the clearance of saxagliptin and its main metabolite on the drug dose and duration of therapy when taking saxagliptin once a day in doses from 2.5 mg to 400 mg for 14 days.
Suction
After oral administration, at least 75% of the administered dose of saxagliptin is absorbed. Food intake did not have a significant effect on the pharmacokinetics of saxagliptin in healthy volunteers. A high-fat meal had no effect on saxagliptin Cmax, whereas AUC increased by 27% compared to fasting. The time to reach Cmax (Tmax) for saxagliptin increased by approximately 0.5 hours when taking the drug with food compared to taking it on an empty stomach. However, these changes are not clinically significant.
Distribution
The binding of saxagliptin and its main metabolite to serum proteins is insignificant, so it can be assumed that the distribution of saxagliptin will not be subject to significant changes due to changes in the protein composition of the blood serum, observed in liver or renal failure.
Metabolism
Saxagliptin is metabolized mainly with the participation of cytochrome P450 3A4/5 isoenzymes (CYP3A4/5) with the formation of an active main metabolite, the inhibitory effect of which on DPP-4 is 2 times less pronounced than that of saxagliptin.
Removal
Saxagliptin is excreted in urine and bile. After a single dose of 50 mg of 14C-labeled saxagliptin, 24% of the dose was excreted by the kidneys as unchanged saxagliptin and 36% as the main metabolite of saxagliptin. The total radioactivity detected in the urine corresponded to 75% of the dose taken. The mean renal clearance of saxagliptin was approximately 230 ml/min, and the mean glomerular filtration rate was approximately 120 ml/min. For the main metabolite, renal clearance was comparable to mean glomerular filtration values. About 22% of the total radioactivity was found in feces.
Pharmacokinetics in special clinical situations
Renal dysfunction
In patients with mild renal failure, the AUC values of saxagliptin and its main metabolite were 1.2 and 1.7 times higher, respectively, than those in individuals with normal renal function. This increase in AUC values is not clinically significant and therefore no dose adjustment is required.
In patients with moderate to severe renal impairment, as well as in patients on hemodialysis, the AUC values of saxagliptin and its main metabolite were 2.1 and 4.5 times higher, respectively, than those in individuals with normal renal function. For patients with moderate to severe renal impairment, as well as for patients on hemodialysis, the dose of saxagliptin should be 2.5 mg once daily.
Liver dysfunction
In patients with mild, moderate and severe hepatic impairment, no clinically significant changes in the pharmacokinetic parameters of saxagliptin were detected, therefore no dose adjustment is required for such patients. Elderly patients In patients 65-80 years old, no clinically significant differences in the pharmacokinetic parameters of saxagliptin were identified compared with younger patients (18-40 years old), so no dose adjustment is required in elderly patients. However, it should be borne in mind that this category of patients is more likely to have decreased renal function
Indications for use of Ongliza 5 mg 30 pcs. film-coated tablets
Type 2 diabetes mellitus in addition to diet and exercise to improve glycemic control as: monotherapy; initial combination therapy with metformin; addition to monotherapy with metformin, thiazolidinediones, sulfonylurea derivatives, insulin (including in combination with metformin) in the absence of adequate glycemic control during this therapy; adding to the combination of metformin and sulfonylurea derivatives in the absence of adequate glycemic control on this therapy.
Contraindications
Increased individual sensitivity to any component of the drug; Serious hypersensitivity reactions (anaphylaxis or angioedema) to DPP-4 inhibitors; Diabetes mellitus type 1 (use not studied); Diabetic ketoacidosis; Congenital galactose intolerance, lactase deficiency and glucose-galactose malabsorption; Pregnancy, lactation; Age up to 18 years (safety and effectiveness have not been studied).
With caution: moderate to severe renal failure; elderly patients; combined use with sulfonylurea derivatives or insulin, patients with a history of pancreatitis (the relationship between taking the drug and an increased risk of developing pancreatitis has not been established).
Application of Ongliza 5 mg 30 pcs. film-coated tablets during pregnancy and breastfeeding
Due to the fact that the use of saxagliptin during pregnancy has not been studied, the drug should not be prescribed during pregnancy. It is not known whether saxagliptin passes into breast milk. Due to the fact that the possibility of saxagliptin passing into breast milk cannot be excluded, breastfeeding should be stopped during treatment with saxagliptin or therapy should be discontinued, taking into account the balance of risk for the child and benefit for the mother.
special instructions
The use of Onglyza® as part of triple therapy with metformin and thiazolidinediones has not been studied.
Patients with impaired renal function
Dose adjustment is recommended for patients with moderate to severe renal failure, as well as for patients on hemodialysis. Before starting therapy and periodically during treatment with the drug, it is recommended to evaluate renal function.
Use in combination with drugs that can cause hypoglycemia
Sulfonylureas and insulin can cause hypoglycemia, therefore, to reduce the risk of hypoglycemia when used concomitantly with Onglyza®, a reduction in the dose of sulfonylureas or insulin may be necessary.
Hypersensitivity reactions
Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported during post-marketing use of saxagliptin. If a serious hypersensitivity reaction develops, discontinue use of the drug, evaluate other possible causes of the phenomenon, and prescribe alternative therapy for diabetes mellitus.
Pancreatitis
As part of the post-marketing use of the drug, spontaneous reports of cases of acute pancreatitis have been received. Patients taking Onglyza® should be informed about the characteristic symptoms of acute pancreatitis: prolonged, intense pain in the abdominal area. If you suspect the development of pancreatitis, you should stop taking Onglyza®. The incidence of protocol-confirmed pancreatitis in the SAVOR study was 0.3% in the saxagliptin and placebo groups in the all randomized patient population.
Elderly patients
Of the 16,492 patients randomized in the SAVOR study, 8,561 patients (51.9%) were 65 years of age or older, and 2,330 patients (14.1%) were 75 years of age or older. Of these, 4290 patients aged 65 years and older and 1169 patients aged 75 years and older received saxagliptin.
According to clinical studies, efficacy and safety rates in patients aged 65 years and older and 75 years and older did not differ from those in younger patients. Saxagliptin and its main metabolite are partially eliminated by the kidneys, so it must be taken into account that elderly patients are more likely to have decreased renal function.
Ongliza® contains lactose. Patients with congenital galactose intolerance, lactase deficiency and glucose-galactose malabsorption should not take this drug.
Overdose
Symptoms of intoxication have not been described with long-term use of the drug in doses up to 80 times higher than recommended. Treatment: in case of overdose, symptomatic therapy should be used. Saxagliptin and its main metabolite are eliminated from the body by hemodialysis (removal rate: 23% of the dose in 4 hours).
Side effects of Ongliza 5 mg 30 pcs. film-coated tablets
Side effects of Ongliza® in glycemic control studies
The table shows the side effects identified in patients with T2DM when taking Onglyza® at a dose of 5 mg during clinical trials. The overall incidence of adverse events when taking Onglyza 5 mg alone and when added to therapy with metformin, thiazolidinedione or glibenclamide was comparable to that in the placebo group. Frequency scale of adverse reactions: very often (>1/10); often (>1/100, 1/1000, 1/10000,
Side effects according to a pooled analysis of five placebo-controlled clinical trials of Ongliza®
Infections and infestations: Often - Upper respiratory tract infections; Common - Urinary tract infections; Often - Gastroenteritis; Often - Sinusitis.
From the gastrointestinal tract: Often - Vomiting.
From the nervous system: Often - Headache.
The incidence of hypersensitivity reactions observed at week 24 of therapy was 1.5% in patients receiving Onglyza 5 mg and 0.4% in patients receiving placebo. Hypersensitivity reactions that occurred in patients taking Ongliza® did not require hospitalization and were assessed by the attending physicians as not life-threatening.
Side effects of Ongliza® in combination therapy in glycemic control studies
In a study of the combined use of saxagliptin and glibenclamide, the incidence of confirmed hypoglycemic episodes was 0.8% in the saxagliptin 5 mg group and 0.7% in the placebo group. The incidence of confirmed hypoglycaemic episodes in patients receiving Onglyza 5 mg in two saxagliptin monotherapy studies, a saxagliptin and metformin combination study, and a saxagliptin and thiazolidinedione combination study was comparable to that observed with placebo.
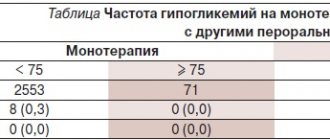
In a study of saxagliptin and insulin, the overall incidence of hypoglycemia was 18.4% in the saxagliptin 5 mg group and 19.9% in the placebo group, while the incidence of confirmed symptomatic hypoglycemia was 5.3% and 3.3 %, respectively.
In a study of saxagliptin in combination with metformin and sulfonylureas, the overall incidence of hypoglycemia was 10.1% in patients receiving Onglyza 5 mg and 6.3% in patients receiving placebo, the incidence of confirmed hypoglycemia was 1.6 % and 0%, respectively.
In a study of saxagliptin with thiazolidinediones, the incidence of peripheral edema was higher in the saxagliptin 5 mg group compared with the placebo group (8.1% and 4.3%, respectively). Peripheral edema was mild or moderate and did not lead to discontinuation of treatment. The incidence of peripheral edema in patients treated with Onglyza 5 mg during clinical trials of saxagliptin monotherapy and combination therapy with metformin or glibenclamide was comparable to that of placebo (1.7% and 2.4%, respectively).
During initial combination therapy with saxagliptin 5 mg and metformin, cases of nasopharyngitis and headache were frequently reported. The incidence of nasopharyngitis was higher with combination therapy (6.9%) compared with monotherapy with saxagliptin 10 mg (4.2%) and metformin (4.0%). Headache was observed more often in the group of patients on combination therapy with metformin and saxagliptin 5 mg (7.5%) compared with the monotherapy groups with saxagliptin 10 mg (6.3%) and metformin (5.2%).
Side effects of Onglyza® in the SAVOR study
In the SAVOR study, 8240 patients received Onglyza 2.5 mg or 5 mg once daily, and 8173 patients received placebo. The average duration of therapy with Onglyza®, regardless of treatment interruptions, was 1.8 years. In 3698 patients (45%), the duration of therapy with Ongliza® was 2-3 years.
The overall incidence of adverse events in this study in the Onglyza group (72.5%) was comparable to the incidence of adverse events in the placebo group (72.2%). The rate of discontinuation of therapy due to adverse events was comparable in patients taking Onglyza® (4.9%) and placebo (5%).
The SAVOR study assessed the effect of Ongliza® on the incidence of cardiovascular events. The addition of Onglyza to therapy has not been shown to increase the risk of cardiovascular events (such as cardiovascular mortality, non-fatal myocardial infarction, non-fatal ischemic stroke) in patients with T2DM compared with placebo (RR 1.00; 95% CI 0.89, 1.12; P
The incidence of per-protocol pancreatitis was 0.3% in the saxagliptin and placebo groups in the all randomized patient population. The incidence of hypersensitivity reactions was 1.1% in the Onglyza and placebo groups.
Hypoglycemia
The overall incidence of hypoglycemia (reported in patient diaries) in the SAVOR study was 17.1% in the Onglyza group and 14.8% in the placebo group. The proportion of patients who experienced severe hypoglycemia during treatment (hypoglycemia requiring third party assistance) was higher in the saxagliptin group compared to the placebo group (2.1% and 1.6%, respectively).
The increased risk of hypoglycemia overall, as well as severe hypoglycemia in the saxagliptin group, was primarily observed in patients receiving sulfonylureas, but not in patients receiving insulin or metformin as background therapy. An increased risk of hypoglycemia overall, as well as severe hypoglycemia, was mainly observed in patients with baseline HbAlc values.
The following side effects have been reported during post-marketing use of saxagliptin: acute pancreatitis and hypersensitivity reactions, including anaphylaxis, angioedema, rash and urticaria. It is impossible to reliably estimate the incidence of these phenomena, since reports were received spontaneously from a population of unknown size.
Laboratory research
In clinical studies, the frequency of changes in laboratory parameters when taking saxagliptin at a dose of 5 mg and placebo was comparable. There was a slight decrease in lymphocyte counts, but the mean absolute lymphocyte count remained stable and within the normal range when taking saxagliptin daily for up to 102 weeks. The decrease in the number of lymphocytes was not accompanied by clinically significant adverse reactions. The clinical significance of the decrease in lymphocyte counts during saxagliptin therapy is not known. In the SAVOR study, a decrease in the number of lymphocytes in the Ongliza® group was noted in 0.5% of patients, in the placebo group - in 0.4% of patients.
Drug interactions
Analysis of clinical trial data suggests that the risk of clinically significant interactions between saxagliptin and other drugs when used together is low. The metabolism of saxagliptin is predominantly mediated by the cytochrome P450 3A4/5 isoenzyme system (CYP3A4/5). In vitro studies have shown that saxagliptin and its main metabolite do not inhibit the isoenzymes CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4 and do not induce the isoenzymes CYP1A2, 2B6, 2C9, and 3A4. In studies involving healthy volunteers, the pharmacokinetic parameters of saxagliptin and its main metabolite were not significantly changed by metformin, glibenclamide, pioglitazone, digoxin, simvastatin, diltiazem, ketoconazole, omeprazole, a combination of aluminum hydroxide, magnesium hydroxide and simethicone, as well as famotidine. Saxagliptin does not significantly change the pharmacokinetic parameters of metformin, glibenclamide, pioglitazone, digoxin, simvastatin, diltiazem, ketoconazole or combined oral (estrogen + progestogen) contraceptives. The effect of inducers of CYP3A4/5 isoenzymes on the pharmacokinetics of saxagliptin has not been studied. However, the combined use of saxagliptin and inducers of CYP3A4/5 isoenzymes, such as carbamazepine, dexamethasone, phenobarbital, phenytoin and rifampicin, may lead to a decrease in the concentration of saxagliptin in plasma and an increase in the concentration of its main metabolite.
The effects of smoking, dietary intake, herbal preparations and alcohol consumption on saxagliptin therapy have not been studied.
How does the time of day affect the absorption of carbohydrates?
Scientists have long noticed that the same product is absorbed differently by the body depending on the time of day. And as it turned out, this is due to the production of hormones, or rather, the predominance of one or another hormone. The fact is that hormones that affect carbohydrate metabolism are produced at a strictly defined time, that is, they have their own daily rhythm. At the same time, some hormones promote the absorption of carbohydrates, while others, on the contrary, suppress this process. And it doesn’t matter what time we wake up or go to bed - hormones do not adapt to us and continue to work in their own way. And, if our nutrition does not coincide with the biological rhythm, then the risk of diabetes increases significantly.
So how can you distribute carbohydrates throughout the day so that your blood sugar doesn't spike? You just need to follow a few simple rules:
- You can indulge in a lot at breakfast The fact is that in the morning, cells absorb glucose least of all due to low sensitivity to insulin. And the culprit is the stress hormone cortisol, which is produced in the early morning hours, helping the body adapt after sleep. But in the future, all sugar not absorbed by cells is converted into a useful supply of energy - glycogen. But! After consuming “medium” and “fast” carbohydrates, a feeling of hunger sets in very quickly. Therefore, if you add protein foods with a sufficient amount of fat to your diet, you will be provided with long-term satiety, and the coming day will allow you to use up all the “eaten” calories. However, for those who are planning a second breakfast (snack), of course, it is better not to overload the first meal with calories.
- At lunch , cortisol still continues to be produced, but in smaller quantities, so you can eat “medium” carbohydrates in the form of side dishes (stewed vegetables, potatoes, cereals, pasta). But it’s better not to eat sweets. Even during the daytime, “long” carbohydrates (salads) and protein foods (meat, fish, poultry) must be present.
- In the evening, insulin replaces cortisol. And all the “fast” and “medium” carbohydrates eaten begin to be instantly absorbed, predisposing to weight gain. Accordingly, the load on the body increases. Therefore, at dinner, it is better to give preference to “long” carbohydrates (fresh vegetables) and protein foods (cottage cheese, kefir, poultry, fish), but with a low fat content.
- Try to your main meal (in terms of calories) either for breakfast or lunch . Eating high-calorie foods in the evening increases the risk of developing diabetes, even in people with ideal body weight. But... a very early breakfast at 4-5 am, as well as a late dinner, can provoke the development of diabetes. In the early morning, the sleep hormone melatonin still continues to be produced, which suppresses insulin production, thereby increasing the risk of developing diabetes. Therefore, if you still have to have breakfast so early, it is better to give preference to dishes high in protein and fat (omelet, boiled eggs, cottage cheese, cheeses, avocado).
- And a little more about sweets: as mentioned earlier, it is preferable to eat “fast” carbohydrates in the morning and in small quantities , and, best of all, after eating foods that contain a large amount of plant fiber . Thanks to “long” carbohydrates, sweets will be absorbed more evenly and without a sharp increase in blood sugar.
- And, lastly, your daily diet should contain such an amount of calories that your body weight remains normal , since with every extra kilogram the risk of developing diabetes increases. If you are still overweight or obese, you should make every effort to lose weight.
Proper nutrition significantly reduces the risk of developing diabetes, but don’t forget about physical activity and quitting smoking!
Ongliza, 5 mg, film-coated tablets, 30 pcs.
In patients with T2DM and healthy volunteers, similar parameters of the pharmacokinetics of saxagliptin and its main metabolite were noted. Saxagliptin is rapidly absorbed after oral administration on an empty stomach, with Cmax of saxagliptin and the major metabolite in plasma achieved within 2 and 4 hours, respectively. With increasing doses of saxagliptin, a proportional increase in the Cmax and AUC of saxagliptin and its main metabolite was noted. After a single 5 mg oral dose of saxagliptin to healthy volunteers, the mean AUC values of saxagliptin and its major metabolite were 78 and 214 ng h/ml, and plasma Cmax values were 24 and 47 ng/ml, respectively.
The mean terminal T1/2 of saxagliptin and its major metabolite was 2.5 and 3.1 hours, respectively, and the mean T1/2 of plasma DPP-4 inhibition was 27 hours. Inhibition of plasma DPP-4 activity for at least 24 h after taking saxagliptin is due to its high affinity for DPP-4 and long-term binding to it. No noticeable accumulation of saxagliptin and its main metabolite was observed during long-term administration of the drug once a day. There was no dependence of the clearance of saxagliptin and its main metabolite on the drug dose and duration of therapy when taking saxagliptin once a day in doses from 2.5 to 400 mg for 14 days.
Suction
After oral administration, at least 75% of the administered dose of saxagliptin is absorbed. Food intake did not have a significant effect on the pharmacokinetics of saxagliptin in healthy volunteers. A high-fat meal had no effect on saxagliptin Cmax, whereas AUC increased by 27% compared to fasting. Tmax for saxagliptin increased by approximately 0.5 hours when the drug was taken with food compared to when taken on an empty stomach. However, these changes are not clinically significant.
Distribution
The binding of saxagliptin and its main metabolite to serum proteins is insignificant, so it can be assumed that the distribution of saxagliptin will not be subject to significant changes due to changes in the protein composition of the blood serum, observed in liver or renal failure.
Metabolism
Saxagliptin is metabolized mainly with the participation of cytochrome P450 isoenzymes CYP3A4/5 with the formation of an active main metabolite, the inhibitory effect of which on DPP-4 is 2 times less pronounced than that of saxagliptin.
Removal
Saxagliptin is excreted in urine and bile. After a single dose of 50 mg of 14C-labeled saxagliptin, 24% of the dose was excreted by the kidneys as unchanged saxagliptin and 36% as the major metabolite of saxagliptin. The total radioactivity detected in the urine corresponded to 75% of the dose taken. The mean renal clearance of saxagliptin was approximately 230 ml/min, and the mean glomerular filtration rate was approximately 120 ml/min. For the main metabolite, renal clearance was comparable to mean glomerular filtration values.
About 22% of the total radioactivity was found in feces.
Pharmacokinetics in special clinical situations
Renal dysfunction.
In patients with mild renal failure, the AUC values of saxagliptin and its main metabolite were 1.2 and 1.7 times higher, respectively, than those in individuals with normal renal function. This increase in AUC values is not clinically significant and therefore no dose adjustment is required.
In patients with moderate to severe renal impairment, as well as in patients on hemodialysis, the AUC values of saxagliptin and its main metabolite were 2.1 and 4.5 times higher, respectively, than those in individuals with normal renal function. For patients with moderate to severe renal impairment, as well as for patients on hemodialysis, the dose of saxagliptin should be 2.5 mg once a day (see “Dosage and Administration”, “Special Instructions”).
Liver dysfunction.
In patients with mild, moderate and severe hepatic impairment, no clinically significant changes in the pharmacokinetic parameters of saxagliptin were identified, therefore no dose adjustment is required for such patients.
Elderly patients.
In patients 65–80 years of age, no clinically significant differences in the pharmacokinetic parameters of saxagliptin were identified compared with younger patients (18–40 years), so dose adjustment is not required in elderly patients. However, it should be borne in mind that in this category of patients, a decrease in renal function is more likely (see “Method of administration and dosage”, “Special instructions”).
Exercise and smoking cessation
According to Russian and foreign diabetic associations, very little is needed to prevent the development of diabetes - just 150 minutes of active physical activity per week. Of course, we are not talking about calm walking, but the type of activity does not matter. The main thing is that the classes give you pleasure (this will prevent you from giving up in the near future) and are regular.
And, if you smoke, quitting will further reduce your risk of developing diabetes. So far, the opinions of scientists about why smoking provokes diabetes are quite contradictory, but the very fact of the influence of smoking on the development of diabetes does not raise any doubts, even despite the fact that smokers, on average, have less body weight.
I hope that the information in this article will help you avoid developing type 2 diabetes. But in order not to miss the onset of the disease, I recommend checking the level of glucose (sugar) in the blood at least once a year. And for those who have risk factors for diabetes, in addition to glucose, it is advisable to check the level of insulin in the blood by calculating the HOMA-IR* index, since this indicator is the most sensitive and often increases long before the development of the disease.
*HOMA-IR index is an indicator that is used to assess the sensitivity of body cells to insulin and reflects the risk of developing type 2 diabetes.
You can find out your risk of developing diabetes and get a detailed consultation with an endocrinologist lasting 1 hour at the Expert medical center. Also in our center there is a “Check-up Diabetes Diagnostics” , which includes all the necessary studies and consultation with an endocrinologist.
Ongliza 5mg n30 tab.
Dosage form
: film-coated tablets
Compound
: One film-coated tablet contains:
Active substances
: saxagliptin 2.5 mg or 5.0 mg in the form of saxagliptin hydrochloride;
Excipients
: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, 1 M hydrochloric acid solution or 1 M sodium hydroxide solution, Opadry II white [polyvinyl alcohol, titanium dioxide, macrogol (PEG 3350), talc], Opadry II yellow [ polyvinyl alcohol, titanium dioxide, macrogol (PEG 3350), talc, dye yellow iron oxide (E172)] (for a dosage of 2.5 mg), Opadry II pink [polyvinyl alcohol, titanium dioxide, macrogol (PEG 3350), talc, dye iron oxide red (E172)] (for a dosage of 5.0 mg), Opacode blue ink*. * Composition of Opacode blue ink: shellac in ethyl alcohol, FD&C Blue #2 / indigo carmine aluminum pigment (E132), n-butyl alcohol, propylene glycol, isopropyl alcohol, 28% ammonium hydroxide. Very small amounts of shellac and FD&C Blue #2/indigo carmine aluminum pigment remain on the tablets when marking is applied. The solvents contained in the ink are removed during the manufacturing process.
Description
:
2.5 mg film-coated tablets
: pale yellow to light yellow, round, biconvex film-coated tablets, debossed with "2.5" on one side and "4214" on the other side, printed in blue.
5 mg film-coated tablets
: round, biconvex, pink film-coated tablets, marked “5” on one side and “4215” on the other side, printed in blue.
Pharmacotherapeutic group
: hypoglycemic agent - dipeptidyl peptidase-4 inhibitor
Code ATX
A10BH03
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
Saxagliptin is a powerful selective reversible competitive inhibitor of dipeptidyl peptidase-4 (DPP-4). In patients with type 2 diabetes mellitus (T2DM), taking saxagliptin leads to suppression of the activity of the DPP-4 enzyme within 24 hours. After oral glucose administration, inhibition of DPP-4 leads to a 2-3 fold increase in the concentration of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), a decrease in the concentration of glucagon and an increase in the glucose-dependent response of beta cells, which leads to an increase in the concentration of insulin and C-peptide. Release of insulin from pancreatic beta cells and decreased release of glucagon from pancreatic alpha cells results in decreased fasting and postprandial glycemia. The efficacy and safety of saxagliptin at doses of 2.5 mg, 5 mg and 10 mg once daily were studied in six double-blind, placebo-controlled studies involving 4148 patients with T2DM. Taking the drug was accompanied by a statistically significant improvement in glycosylated hemoglobin (HbAlc), fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) compared to the control. Patients in whom the target glycemic level could not be achieved while taking saxagliptin as monotherapy were additionally prescribed metformin, glibenclamide or thiazolidinediones. When taking saxagliptin at a dose of 5 mg, a decrease in HbAlc was noted after 4 weeks and FPG after 2 weeks. In the group of patients receiving saxagliptin in combination with metformin, glibenclamide or thiazolidinediones, a decrease in HbAlc was also observed after 4 weeks and FPG after 2 weeks. The effect of saxagliptin on the lipid profile was similar to that of placebo. No weight gain was observed during saxagliptin therapy.
Pharmacokinetics
In patients with T2DM and healthy volunteers, similar parameters of the pharmacokinetics of saxagliptin and its main metabolite were noted.
Saxagliptin is rapidly absorbed after oral administration on an empty stomach, with maximum plasma concentrations of saxagliptin and the major metabolite (Cmax) achieved within 2 hours and 4 hours, respectively. With an increase in the dose of saxagliptin, a proportional increase in Cmax and the area under the concentration-time curve (AUC) of saxagliptin and its main metabolite was noted. Following a single 5 mg oral dose of saxagliptin to healthy volunteers, the mean AUC values of saxagliptin and its major metabolite were 78 ngch/ml and 214 ngh/ml, and plasma Cmax values were 24 ng/ml and 47 ng/ml, respectively. The mean terminal half-life (t½) of saxagliptin and its major metabolite was 2.5 hours and 3.1 hours, respectively, and the mean t½ of plasma DPP-4 inhibition was 26.9 hours. Inhibition of plasma DPP-4 activity for, at least 24 hours after taking saxagliptin due to its high affinity for DPP-4 and prolonged binding to it. No noticeable accumulation of saxagliptin and its main metabolite was observed during long-term administration of the drug once a day. There was no dependence of the clearance of saxagliptin and its main metabolite on the drug dose and duration of therapy when taking saxagliptin once a day in doses from 2.5 mg to 400 mg for 14 days. Absorption
After oral administration, at least 75% of the administered dose of saxagliptin is absorbed.
Food intake did not have a significant effect on the pharmacokinetics of saxagliptin in healthy volunteers. A high-fat meal had no effect on saxagliptin Cmax, whereas AUC increased by 27% compared to fasting. The time to reach Cmax (Tmax) for saxagliptin increased by approximately 0.5 hours when taking the drug with food compared to taking it on an empty stomach. However, these changes are not clinically significant. Distribution
The binding of saxagliptin and its main metabolite to serum proteins is insignificant, so it can be assumed that the distribution of saxagliptin will not be subject to significant changes due to changes in the protein composition of the blood serum, observed in liver or renal failure.
Metabolism
Saxagliptin is metabolized mainly with the participation of cytochrome P450 3A4/5 isoenzymes (CYP3A4/5) with the formation of an active main metabolite, the inhibitory effect of which on DPP-4 is 2 times less pronounced than that of saxagliptin.
Excretion
Saxagliptin is excreted in the urine and bile.
After a single dose of 50 mg of 14C-labeled saxagliptin, 24% of the dose was excreted by the kidneys as unchanged saxagliptin and 36% as the main metabolite of saxagliptin. The total radioactivity detected in the urine corresponded to 75% of the dose taken. The mean renal clearance of saxagliptin was approximately 230 ml/min, and the mean glomerular filtration rate was approximately 120 ml/min. For the main metabolite, renal clearance was comparable to mean glomerular filtration values. About 22% of the total radioactivity was found in feces. Pharmacokinetics in special clinical situations Impaired renal function
In patients with mild renal failure, the AUC values of saxagliptin and its main metabolite were 1.2 and 1.7 times higher, respectively, than those in individuals with normal renal function.
This increase in AUC values is not clinically significant and therefore no dose adjustment is required. In patients with moderate to severe renal impairment, as well as in patients on hemodialysis, the AUC values of saxagliptin and its main metabolite were 2.1 and 4.5 times higher, respectively, than those in individuals with normal renal function. For patients with moderate to severe renal impairment, as well as for patients on hemodialysis, the dose of saxagliptin should be 2.5 mg once a day (see sections “Dosage and Administration” and “Special Instructions”). Hepatic impairment
In patients with mild, moderate and severe hepatic impairment, no clinically significant changes in the pharmacokinetic parameters of saxagliptin were identified, therefore no dose adjustment is required for such patients.
Elderly patients
In patients 65-80 years old, no clinically significant differences in the pharmacokinetic parameters of saxagliptin were identified compared with younger patients (18-40 years old), so no dose adjustment is required in elderly patients. However, it should be borne in mind that in this category of patients, a decrease in renal function is more likely (see sections “Dosage and Administration” and “Special Instructions”).
INDICATIONS
Type 2 diabetes mellitus in addition to diet and exercise to improve glycemic control as:
- monotherapy;
- initial combination therapy with metformin;
- addition to monotherapy with metformin, thiazolidinediones, sulfonylurea derivatives, in the absence of adequate glycemic control on this therapy.
CONTRAINDICATIONS
- Increased individual sensitivity to any component of the drug;
- Diabetes mellitus type 1 (use not studied);
- Use in combination with insulin (not studied);
- Diabetic ketoacidosis;
- Congenital galactose intolerance, lactase deficiency and glucose-galactose malabsorption;
- Pregnancy, lactation;
- Age up to 18 years (safety and effectiveness have not been studied).
Carefully
: moderate to severe renal failure; elderly patients; combined use with sulfonylurea derivatives.
DURING PREGNANCY AND BREASTFEEDING
Due to the fact that the use of saxagliptin during pregnancy has not been studied, the drug should not be prescribed during pregnancy. It is not known whether saxagliptin passes into breast milk. Due to the fact that the possibility of saxagliptin passing into breast milk cannot be excluded, breastfeeding should be stopped during treatment with saxagliptin or therapy should be discontinued, taking into account the balance of risk for the child and benefit for the mother.
METHOD OF APPLICATION AND DOSES
Inside, regardless of food intake.
Monotherapy
: the recommended dose of saxagliptin is 5 mg once daily.
Combination therapy
: the recommended dose of saxagliptin is 5 mg once a day in combination with metformin, thiazolidinediones or sulfonylurea derivatives.
When starting combination therapy with metformin,
the recommended dose of saxagliptin is 5 mg once a day, the initial dose of metformin is 500 mg per day.
In case of inadequate response, the dose of metformin may be increased. If you miss taking Ongliza®, the missed tablet should be taken as soon as the patient remembers, but you should not take a double dose of the drug within one day. Use in special groups of patients Patients with impaired renal function
For patients with mild renal failure (creatinine clearance >50 ml/min), no dose adjustment is required.
For patients with moderate or severe renal failure (creatinine clearance <50 ml/min), as well as for patients on hemodialysis, the recommended dose of Onglyza® is 2.5 mg once daily. The drug should be taken at the end of the hemodialysis session. The use of saxagliptin in patients on peritoneal dialysis has not been studied. It is recommended that renal function be assessed before initiating saxagliptin therapy and during treatment. Patients with impaired liver function
No dose adjustment is required for mild, moderate and severe liver dysfunction.
Elderly patients
No dose adjustment is required in elderly patients.
However, when choosing a dose, it should be taken into account that in this category of patients a decrease in renal function is more likely. Children
The safety and effectiveness of the drug in patients under 18 years of age have not been studied.
Concomitant use with strong CYP3A4/5 inhibitors
When used concomitantly with strong CYP3A4/5 inhibitors, such as ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir and telithromycin, the recommended dose of Onglyza® is 2.5 mg 1 time per day.
SIDE EFFECT
The table shows the side effects identified in patients with T2DM when taking Onglyza® at a dose of 5 mg during clinical trials. The overall incidence of adverse events when taking Onglyza 5 mg alone and when added to therapy with metformin, thiazolidinedione or glibenclamide was comparable to that in the placebo group. Frequency scale of adverse reactions: very often (>1/10); often (>1/100, <1/10); uncommon (>1/1000, <1/100); rare (>1/10000, <1/1000); very rare (<1/10000).
Table 1 Side effects according to a pooled analysis of five placebo-controlled clinical trials of Ongliza®
| Infections and infestations | |
| Upper respiratory tract infections | Often |
| Urinary tract infections | Often |
| Gastroenteritis | Often |
| Sinusitis | Often |
| From the gastrointestinal tract | |
| Vomit | Often |
| From the nervous system | |
| Headache | Often |
Side effects of Ongliza® in combination therapy
In a study of the combined use of saxagliptin and glibenclamide, the frequency of confirmed episodes of hypoglycemia in the saxagliptin 5 mg (0.8%) and placebo (0.7%) groups was not statistically different.
The incidence of confirmed hypoglycemic episodes in patients receiving Onglyza 5 mg in two saxagliptin monotherapy studies, a saxagliptin and metformin combination study, and a saxagliptin and thiazolidinedione combination study was comparable to that observed with placebo. In a study of saxagliptin with thiazolidinediones, the incidence of peripheral edema was higher in the saxagliptin 5 mg group compared with the placebo group (8.1% and 4.3%, respectively). Peripheral edema was mild or moderate and did not lead to discontinuation of treatment. The incidence of peripheral edema in patients treated with Onglyza 5 mg during clinical trials of saxagliptin monotherapy and combination therapy with metformin or glibenclamide was comparable to that of placebo (1.7% and 2.4%, respectively). During initial combination therapy with saxagliptin 5 mg and metformin, cases of nasopharyngitis and headache were frequently reported. The incidence of nasopharyngitis was higher with combination therapy (6.9%) compared with monotherapy with saxagliptin 10 mg (4.2%) and metformin (4.0%). Headache was more common in the group of patients on combination therapy with metformin and saxagliptin 5 mg (7.5%) compared with the monotherapy groups with saxagliptin 10 mg (6.3%) and metformin (5.2%). Laboratory studies
In clinical studies, the frequency of changes in laboratory parameters when taking saxagliptin at a dose of 5 mg and placebo was comparable. There was a slight decrease in lymphocyte counts, but the mean absolute lymphocyte count remained stable and within the normal range when taking saxagliptin daily for up to 102 weeks. The decrease in the number of lymphocytes was not accompanied by clinically significant adverse reactions. The clinical significance of the decrease in lymphocyte counts during saxagliptin therapy is not known.
OVERDOSE
Symptoms of intoxication have not been described with long-term use of the drug in doses up to 80 times higher than recommended. In case of overdose, symptomatic therapy should be used. Saxagliptin and its main metabolite are eliminated from the body by hemodialysis (removal rate: 23% of the dose in 4 hours).
INTERACTIONS WITH OTHER MEDICINES AND OTHER TYPES OF INTERACTIONS
Analysis of clinical trial data suggests that the risk of clinically significant interactions between saxagliptin and other drugs when used together is low. The metabolism of saxagliptin is predominantly mediated by the cytochrome P450 3A4/5 isoenzyme system (CYP3A4/5). In vitro studies have shown that saxagliptin and its main metabolite do not inhibit the isoenzymes CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1 and 3A4 and do not induce the isoenzymes CYP1A2, 2B6, 2C9, and 3A4. In studies involving healthy volunteers, the pharmacokinetic parameters of saxagliptin and its main metabolite were not significantly changed by metformin, glibenclamide, pioglitazone, digoxin, simvastatin, diltiazem, ketoconazole, omeprazole, a combination of aluminum hydroxide, magnesium hydroxide and simethicone, as well as famotidine. Saxagliptin does not significantly change the pharmacokinetic parameters of metformin, glibenclamide, pioglitazone, digoxin, simvastatin, diltiazem or ketoconazole. The effect of inducers of CYP3A4/5 isoenzymes on the pharmacokinetics of saxagliptin has not been studied. However, the combined use of saxagliptin and inducers of CYP3A4/5 isoenzymes, such as carbamazepine, dexamethasone, phenobarbital, phenytoin and rifampicin, may lead to a decrease in the concentration of saxagliptin in plasma and an increase in the concentration of its main metabolite. The effects of smoking, dietary intake, herbal preparations and alcohol consumption on saxagliptin therapy have not been studied.
SPECIAL INSTRUCTIONS
The use of Ongliza® in combination with insulin, as well as as part of triple therapy with metformin and thiazolidinediones or metformin and sulfonylurea derivatives, has not been studied.
Patients with impaired renal function
Dose adjustment is recommended for patients with moderate to severe renal impairment, as well as for patients on hemodialysis.
Before starting therapy and periodically during treatment with the drug, it is recommended to evaluate renal function. Use in combination with drugs that can cause hypoglycemia
Sulfonylureas can cause hypoglycemia, therefore, to reduce the risk of hypoglycemia when used simultaneously with Onglyza®, a reduction in the dose of sulfonylureas may be necessary.
Hypersensitivity reactions
The drug should not be prescribed to patients who have had serious hypersensitivity reactions when using other D1111-4 inhibitors.
Elderly patients
According to clinical studies, efficacy and safety rates in patients aged 65 years and older did not differ from those in younger patients. However, increased individual sensitivity to saxagliptin in some elderly patients cannot be excluded. Saxagliptin and its main metabolite are partially eliminated by the kidneys, so it must be taken into account that elderly patients are more likely to have decreased renal function. Ongliza® contains lactose. Patients with congenital galactose intolerance, lactase deficiency and glucose-galactose malabsorption should not take this drug.
INFLUENCE ON THE ABILITY TO DRIVE VEHICLES AND OPERATE MACHINES
No studies have been conducted to study the effect of saxagliptin on the ability to drive vehicles and operate machinery. Please note that saxagliptin may cause dizziness.
RELEASE FORM
Film-coated tablets 2.5 mg and 5 mg. 10 tablets in an aluminum foil blister; 3 blisters in a cardboard box with instructions for use. The blister has a perforation, dividing it into 10 rectangular zones according to the number of tablets.
STORAGE CONDITIONS
At a temperature not higher than 30°C. Keep out of the reach of children.
BEST BEFORE DATE
3 years. Do not use after the expiration date stated on the package.
CONDITIONS OF VACATION FROM PHARMACIES
On prescription.