Rituximab is a revolutionary chemotherapy for some malignant lymphomas, and also produced using genetic engineering.
- Chemical properties and composition
- pharmachologic effect
- Pharmacodynamics and pharmacokinetics
- Indications for use
- Contraindications
- Side effects
- special instructions
- Overdose
- Interaction
- Fertility, pregnancy and lactation
- Best before date
- Analogs
Chemical properties and composition
Rituximab refers to biological drugs, and specifically to monoclonal antibodies or MAbs for short.
This is an immunoglobulin, that is, a large protein synthesized by immune cells.
Functionally, it is an antibody that in the human body must contact a certain type of antigen - a foreign protein, a bacterial product or a certain kind of cellular substrate. Rituximab needs to bind to a receptor on the surface of the unhealthy blood cell.
This is a monoclonal antibody because it is not produced by all blood cells, but by a specific group or clone.
The protein consists of three different length chains of amino acids synthesized by mouse and human cells, so the antibody is called chimeric, that is, mixed from animal and human proteins, to reduce the toxicity of the drug.
pharmachologic effect
In the human body, rituximab looks for a special antigen, CD20, which exists on the surface of most malignant cells that make up B lymphomas and on ordinary B lymphocytes. There should not be such an antigen on other blood cells and it does not float in the blood plasma.
Having contacted the antigen, rituximab breaks off the vital chain of cellular biochemical reactions, and as a result, the cell turns on the program of suicide or apoptosis. In addition, the drug enhances the damaging effects of other cytostatics, so it is included in chemotherapy regimens for lymphomas and lymphocytic leukemia.
Since MAB also kills normal B lymphocytes that have CD20, they also die. As we can see, the selectivity of the drug does not lie in the ability to find and destroy a tumor cell, but in the killing of cells possessing the antigen. In one in a hundred patients with lymphoma, the body produces antibodies that neutralize the chimeric proteins, which makes the use of the drug useless.
Mabthera (rituximab) in the treatment of prognostically unfavorable variants of B-cell lymphomas in children
Non-Hodgkin's lymphomas (NHL) account for about 12% of malignant tumors of childhood and are in third place in the structure of cancer incidence in children after acute leukemia and tumors of the central nervous system (CNS) [1]. Approximately 60% of all NHLs are peripheral B-cell tumors (B-NHL), which in childhood include Burkitt's lymphoma (LB), diffuse large B-cell lymphoma (DLBCL), and primary mediastinal (thymic) large B-cell lymphoma (PLBCL). [2]. These tumors originate from mature B cells and are characterized by varied clinical manifestations with involvement of nodal and extranodal organs, rapid dissemination and an extremely aggressive clinical course [3].
Table 1. Characteristics of children with B-NHL
Rice. 1. Event-free survival of children with prognostically unfavorable variants of B-NHL depending on inclusion in the MabThera (rituximab) chemotherapy program (p = 0.09)
Modern polychemotherapy (PCT) programs, including dexamethasone, methotrexate, cyclophosphamide, Vepesid, cytosar, vincristine and anthracycline antibiotics, allow recovery in 95–100% of patients with initial stages and 1–2 prognostic risk groups [4], while while in stages III–IV and prognostically unfavorable risk groups 3–4, treatment is less effective - event-free survival (EFS) is about 70% [5], which dictates the need to search for new drugs to improve PCT programs for prognostically unfavorable variants of B-NHL . One of these drugs was Mabthera (rituximab) (F. Hoffman - La Roche Ltd, Switzerland), the use of which together with PCT (immunochemotherapy) significantly improved EFS rates in B-NHL in adult patients [6].
Rituximab is a chimeric anti-CD20 monoclonal antibody that consists of a constant (human) and variable (mouse) region. This structure ensures highly specific binding of the drug to CD20-positive cells and low immunogenicity. The target of the drug is the transmembrane antigen CD20, which is involved in the transport of calcium ions across the cell membrane and the differentiation of B cells. During the ontogeny of a B lymphocyte, CD20 appears at the pre-B cell stage and disappears at the stage of plasma cell formation [7].
The mechanism of action of MabThera (rituximab) is due to the activation of complement components (complement-dependent cytotoxicity), antibody-dependent cytotoxicity, induction of apoptosis, and direct antiproliferative effect. In addition, rituximab has a synergistic effect with glucocorticosteroids and anthracycline antibiotics [8].
The maximum concentration of MabThera (rituximab) in blood plasma is 465 mg/l, and the half-life is 8.6 days. Plasma clearance reaches 0.038 l/h. Traces of MabThera (rituximab) can be detected in the body even after 6 months. after its introduction [9].
Historically, the development of PCT regimens in the treatment of B-NHL followed the path of escalation of chemotherapy doses, which resulted in an increase in overall and EFS rates [10]. Currently, PCT for B-NHL is multi-agent in nature, and an important condition for successful treatment is compliance with intervals (timing) between PCT blocks [11]. Considering the relatively low EFS rates in prognostically unfavorable risk groups in children with B-NHL, immunochemotherapy with MabThera (rituximab) has been proposed for this category of patients.
At the present stage in pediatric oncohematology, treatment regimens with MabThera (rituximab) are just beginning to be developed. It is proposed to use MabThera in a therapeutic “window” mode (5 days before the start of the main course of chemotherapy) [12], as well as immediately before the start of each block of chemotherapy, and the possibility of including rituximab in maintenance therapy programs is being studied [13].
Materials and methods
The study of the DOG RORC included 38 children who, from October 1999 to February 2010, were first diagnosed with B-NHL, advanced stage (III–IV stage), prognostically unfavorable (3–4th group) group risk. 18 children made up the comparison group, who were treated according to the B-NHL-BFM95 protocol, and Mabthera (rituximab) was added to the treatment program for children in the main group (n = 20) to the B-NHL-BFM95 protocol, which was administered on day 0 each block of PCT at a dose of 375 mg/m2. According to the PCT program B-NHL-BFM95, in the 3rd prognostic risk group, 5 blocks of PCT are carried out, including dexamethasone, cytosar, vincristine, Vepesid, high doses of methotrexate, cyclophosphamide, doxorubicin (AA-BB-CC-AA-BB), while in group 4 – 6 blocks (AA-BB-SS-AA-BB-SS). A total of 112 injections of MabThera (rituximab) were performed (5 injections for risk group 3, and 6 injections for risk group 4).
The stage of the tumor process was established according to the S. Murhpy classification [14], and the prognostic risk group was determined according to the criteria developed by the German group of BFM researchers [15]. The prevalence of the tumor process was assessed using radiation diagnostic methods (ultrasound, X-ray computed tomography, and in some cases magnetic resonance imaging) and radioisotope methods (67Ga and 99Tc). Statistical data processing was carried out using the SRSS10 program.
Results and discussion
According to the WHO classification of tumors of hematopoietic and lymphoid tissues (2008) [16], based on morpho-immunological and, in some cases, cytogenetic studies of tumor tissue, among all patients with B-NHL (38 children), LB was diagnosed in 76%, DLBCL in 10 % and PMBCL – in 14% of patients. The condition of the children upon admission was severe and extremely severe, which was due to severe lymphoproliferative syndrome, intoxication, cachexia, and acute renal failure caused by spontaneous tumor lysis syndrome. When the tumor was localized in the mediastinum, difficulty breathing was noted due to compression of the trachea by the tumor and severe superior vena cava syndrome. In most patients, the tumor size exceeded 10 cm, in half of the patients tumor ascites was detected, in 20% - pleurisy, in 30% of cases there was bone marrow damage, in 15% of patients - in the central nervous system. During the prephase (dexamethasone and cyclophosphamide) of the B-NHL-BFM95 protocol, acute tumor lysis syndrome developed, requiring hemodiafiltration in 20% of patients in the main group and in 11.2% of children in the comparison group.
In general, the main group and the comparison group were comparable in terms of gender, age, morpho-immunological variant of B-NHL, stages and risk groups (Table 1).
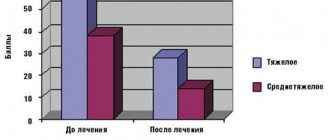
Complete remission (CR) was achieved in 100% of patients receiving MabThera, the EFS rate in this group was 93.10 +/- 6.78% (median follow-up 41.32 +/- 3.14 months). In the comparison group, CR was obtained in 72% of patients, partial remission (PR) in 20%. It should be noted that all cases of PR in prognostically unfavorable risk groups of B-NHL resulted in tumor progression and EFS was 72.2 +/- 10.56% (median follow-up 103.78 +/- 14.6 months) (Fig. 1 ).
Rituximab was well tolerated. Adverse reactions were observed, as a rule, during the first administration of the drug in 8 (40%) children and were represented by urticaria, skin itching and, in one case, bronchospasm. These reactions were quickly stopped by the administration of antihistamines; additional administration of glucocorticoids was required in 2 (10%) patients. There were no adverse reactions with subsequent administrations of rituximab.
The children tolerated chemotherapy satisfactorily. The duration of circulation of methotrexate after its administration in high doses (5000 mg/m2) did not increase in both analyzed groups of patients and amounted to 66–72 hours. Infectious complications were observed to varying degrees in all patients. Thus, grade II–IV mucositis after PCT with methotrexate was detected in 100% of children in both groups. The duration of myelotoxic agranulocytosis (MTA) ranged from 3 to 7 days, the longest was MTA after a CC block including cytosar (3000 mg/m2 x 2 times a day). When assessing the safety profile of therapy, it turned out that the frequency of febrile neutropenia, hematological toxicity and infectious complications were comparable in both groups of patients.
Thus, immunochemotherapy according to the B-NHL-BFM95 program with MabThera (rituximab) in conditions of adequate accompanying therapy allowed to increase the number of complete remissions to 100% against 72% in the comparison group, as well as improve the survival rates of children with extremely unfavorable risk groups and late stages V-NHL. The inclusion of MabThera (rituximab) did not lead to an increase in the incidence of infectious complications or increased toxicity of therapy. It should be assumed that further research aimed at studying the molecular genetic basis of lymphomagenesis will identify new targets for targeted therapy for NHL in children, individualize treatment and increase the survival rate of most patients. Currently, we can recommend the use of MabThera in combination with chemotherapy in this group of patients.
Pharmacodynamics and pharmacokinetics
Almost all of the administered rituximab reaches the site of application of force in the blood, which is designated as 100% bioavailability. The concentration of the drug in the blood increases with each administration, by the fourth dropper the amount of active substance is twice as large as the initial one, and by the eighth dropper the maximum is reached. This process is not affected by the patient’s age, gender, or nationality.
It will take more than three weeks to remove half the dose of the drug from the body, but traces are found six months after completion of treatment. The elimination of rituximab is entrusted to macrophages, which also ingest bacteria, viruses and everything foreign to humans, but this is only an assumption. The kidneys and liver do not appear to be involved in the process of removing the drug; in any case, in patients with insufficient liver function, there is no need to change the dosage.
Medical Internet conferences
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common diseases in the field of oncohematology. Every year, 11.5% of cases of CLL are registered in the world out of the total number of cases of hematological malignancies [1]. This is the most common type of leukemia in Europe and North America. In these countries, it accounts for about 30% of all leukemias. [2]. The five-year survival rate is approximately 70% [1]. The annual incidence of CLL is 3–3.5 per 100,000 thousand population, increasing to 20 per 100,000 for people over 65 and to 50 per 100,000 after 70 years. Thanks to modern therapy, the life expectancy of patients with CLL is steadily increasing and currently often reaches 20–25 years [1].
CLL is mainly a disease of older people. More than 70% become ill over the age of 60 years, the average age of those affected is 65–69 years. Less than 10% develop the disease before the age of 40. [1].
Clinical picture and diagnosis
The clinical picture and course of the disease are very diverse. Some patients experience a smoldering process of disease development and this group does not require therapy for decades. The other part, on the contrary, requires immediate initiation of treatment [3].
The diagnosis of CLL is described in detail by the International CLL Study Group [4]. To make a diagnosis of CLL, a blood test and immunophenotypic study must be performed. The presence of at least 5000/μl B-lymphocytes in the peripheral blood with a characteristic immunophenotype, of which the most prognostically important are the expression of the CD 5 antigen (CLL cells) and B-cell markers CD 19, CD 20, CD 5, CD 23, allows the diagnosis of CLL to be made . [5]. When using the fluorescent hybridization method, at least one chromosomal abnormality is detected in this group of patients, including deletion of 17p (8%) and 11q (15%) [1], which is an unfavorable prognosis factor.
Classification
To determine the stage of the disease, the classification of KR Rai et al is used throughout the world. (1975), reflecting the course of CLL and dividing it into stages depending on clinical manifestations. Another classification of CLL, published in 1981 by JL Binet et al., is based on the level of hemoglobin and platelets in the peripheral blood combined with the number of affected lymphatic areas [5].
Classification of CLL according to A.I. Vorobyov (2007)
| Stages of chronic lymphocytic leukemia according to Rai (1975) | Stages of chronic lymphocytic leukemia according to Binet (1981) | ||
| Stage 0 | Only lymphocytosis in the blood is more than 15.0•109/l, in the bone marrow - more than 40%; prognosis is good, life expectancy corresponds to the population | Stage A | Hemoglobin content more than 100 g/l, platelets more than 100•109/l, enlarged lymph nodes in 1–2 areas; median survival rate is the same as in the population. |
| Stage I | Lymphocytosis is combined with enlarged lymph nodes; prognosis – intermediate, median survival – 9 years | Stage B | The content of hemoglobins and platelets is higher than the same indicators, but the lymph nodes are enlarged in 3 or more areas; median survival is 7 years. |
| Stage II | Lymphocytosis + splenomegaly and (or) liver enlargement, regardless of the size of the lymph nodes; prognosis – intermediate, median survival – 6 years | _ | _ |
| Stage III | Lymphocytosis and a decrease in hemoglobin level less than 110 g/l, regardless of enlargement of the lymph nodes, spleen, liver; prognosis – poor, median survival – less than 3 years | Stage C | Hemoglobin content is less than 100 g/l, platelets - less than 100•109/l in any number of areas with enlarged nodes and regardless of the enlargement of the spleen and liver; median survival is 2 years. |
| Stage IV | Lymphocytosis plus thrombocytopenia below 100•109/l, regardless of anemia and the size of the lymph nodes, spleen and liver; prognosis – poor, median survival – 1.5 years | _ | _ |
Treatment of CLL
The group of patients who have stage A according to JL Binet at the onset of the disease do not require therapy for many years; observation tactics are preferable until signs of indications for starting therapy appear.
There are the following clear indications for starting therapy:
1. signs of intoxication (weight loss of 10% over 6 months, night sweats, low-grade fever without signs of infection)
2. increasing anemia or thrombocytopenia
3. splenomegaly (> 6 cm below the costal margin)
4. massive lymphadenopathy
5. increase in lymphocytosis >2 times in 2 months.
The standard first-line therapy for healthy patients is the FCR regimen (fludarabine, cyclophosphamide, rituximab); in the presence of contraindications to fludarabine therapy, the BR regimen (bendamustine, rituximab) is optimal [5].
According to Russian clinical guidelines for the diagnosis and treatment of lymphoproliferative diseases, 2nd line therapy involves FCR, R-HDMP, BR, FCR-lite regimens. The choice of regimen is based on the first-line therapy used, the time of relapse, and the clinical picture. The choice of the 3rd and subsequent lines is not regulated by these recommendations.
Characteristics of patients and treatment method
Taking into account the above, as well as literature data, we identified and treated 5 patients with relapsed CLL.
The age of the patients ranged from 45 to 67 years, the average age was 59-+2 years. On average, patients were in complete remission for 2.5 years. Each of them received fludarabine-containing regimens as 1st line of therapy.
Before starting treatment, all patients underwent standard methods for diagnosing relapse of CLL: complete blood count, immunophenotyping of bone marrow lymphocytes - high expression of CD 19, CD 20, CD 5, CD 23 was revealed. Additional examination methods were also carried out: CT scan of the neck, thoracic and abdominal organs , small pelvis, according to the results of which all patients had severe lymphadenopathy, hepato- and splenomegaly. Biochemical analysis of blood serum, echocardiography. No pronounced pathology of organs and systems was identified. 2 patients had anemia (80 and 95 g/l)
As a second-line polychemotherapy regimen, patients received 6 courses of BR (bendamustine, rituximab) on an outpatient basis. The dose of bendamustine was 70 mg/m2 on days 1 and 2 of the 28-day cycle. Rituximab was used at a dosage of 375 mg/m2 on day 1 of cycle 1, then 500 mg/m2 on day 1 of each subsequent cycle. The administration of bendamustine was accompanied by infusion therapy in a volume of 1500 ml, rituximab 1000 ml.
Antiemetogenic drugs and glucocorticosteroids were used as premedication 30 minutes before the start of bendamustine administration. In accompanying therapy, drugs that affect tumor lysis syndrome and hepatoprotectors were prescribed.
The duration of bendamustine infusion was 1 hour, and rituximab infusion averaged 4 hours. No adverse reactions were detected during administration. The dose of drugs was not reduced.
After the 3rd course of therapy, patients again underwent a CT scan of the neck, chest, abdominal cavity, and pelvis, according to the results of which all patients showed positive dynamics in the form of a reduction in the size of the tumor mass by more than 50% (in 4 patients) and the absence of new targeted lymphatic nodes, which allowed us to note a partial response to therapy in 4 patients. Stabilization of the process was observed in 1 patient.
When analyzing laboratory data in 2 patients after the 2nd course of therapy, blood counts worsened, anemia (up to 50 g/l) and thrombocytopenia (4 million) increased, and therefore replacement therapy with blood components was carried out. In 3 patients, after 4 courses, severe leukocytopenia (up to 3.5 thousand) was observed, which required the introduction of a colony-stimulating factor. Against the background of agranulocytosis, 1 patient developed polysegmental pneumonia, which was managed with broad-spectrum antibiotics in a hospital setting.
After completing 6 courses of therapy and assessing the response to therapy, all patients experienced clinical and hematological remission. The duration of observation of disease-free survival to date has averaged 4 months.
Conclusions:
- After analyzing the data obtained, we can conclude that it is possible to conduct a course of BR on an outpatient basis, which leads to a reduction in in-hospital complications and reduces the cost of a patient’s hospital stay.
- The 100% remission achieved demonstrates the effectiveness of using this regimen in the amount of 6 courses of therapy in the proposed dosages of drugs.
- Further monitoring of the duration of relapse-free and overall survival of patients is necessary in order to assess the effectiveness of therapy.
Indications for use
Only cell colonies containing CD20 receptors on their surface are sensitive to rituximab, and today such lymphoproliferative processes are specifically detected by tests. As a rule, non-Hodgkin's large B-cell lymphomas and low-aggressive follicular lymphomas respond well to therapy. The drug is used both at the very beginning and for maintenance treatment, and when the disease progresses on other regimens, and when relapses occur.
The second indication is chronic lymphocytic leukemia, used in the first line, in case of ineffectiveness of previous chemotherapy or relapse, usually in combination with other cytostatics.
Contraindications
The use of rituximab if you are allergic to it is excluded.
It should not be used in patients with immune deficiency because the drug destroys already insufficient B lymphocytes.
In acute inflammatory and infectious processes, a temporary taboo is placed on treatment, since suppression of the immune system can lead to generalization of the infection.
The medicine is used very carefully when the levels of leukocytes - neutrophils and platelets are reduced, which is usually due to the consequences of chemotherapy.
The administration of the drug is often complicated by shortness of breath and swelling of the lung tissue, so patients with pulmonary metastases or respiratory failure can have very unpleasant health consequences. However, this is not considered an absolute contraindication; treatment simply needs to be carried out with a certain amount of caution.
Clinical studies are not conducted in minors, so the pitfalls of therapy are unknown, which is why the drug is not licensed for this age category. Do not use in pregnant or lactating mothers.
Prospects for the use of rituximab in human autoimmune diseases
Among the various immune disorders underlying the development of autoimmune diseases, the study of defects in B-cell regulation is of particular interest, including from the point of view of developing new pathogenetically based approaches to treatment [1]. Let us recall that B lymphocytes are cells of the immune system involved in the development and maintenance of adaptive immunity, are formed from hematopoietic precursors in the bone marrow throughout a person’s life, and are involved in maintaining immunological tolerance to one’s own antigens (autoantigens). A defect in B-cell tolerance leads to the synthesis of autoantibodies, which, by activating the effector components of the immune response, induce the development of inflammation and destruction of tissues of the human body. However, the importance of B cells in the development of autoimmune diseases is not limited to the synthesis of “pathogenic” autoantibodies. It has been established that disruption of B-cell co-stimulation of T-lymphocytes plays a fundamental role in the development of autoimmune reactions and can develop at the earliest stages of the pathological process before clinical manifestation and disease [2]. In addition, according to clinical and epidemiological studies, patients with autoimmune rheumatic diseases have an increased risk of developing B-cell non-Hodgkin lymphomas [3]. All this taken together makes B cells promising therapeutic targets for autoimmune diseases [4–7]. The first and so far the only anti-B cell drug approved for use in clinical practice is rituximab (Rituximab, MabThera F. Hoffman-La Roche Ltd.), a chimeric monoclonal antibody to the CD20 antigen of B lymphocytes [8]. The drug has been used in medicine since 1997 for the treatment of B-cell non-Hodgkin lymphomas, and in recent years - a wide range of autoimmune diseases [9]. The choice of the CD20 molecule as a target for monoclonal antibodies is associated with the characteristics of the differentiation of B cells, which, in the process of maturation from stem cells into plasma cells, go through several successive stages, each of which is characterized by the expression of certain membrane molecules (Fig. 1). CD20 expression is observed on the membrane of “early” and mature B lymphocytes, but not on stem cells, “early” pre-B cells, dendritic and plasma cells. Therefore, their depletion does not cancel the regeneration of the pool of B lymphocytes and does not affect the synthesis of immunoglobulins by plasma cells. In addition, CD20 is not released from the membrane of B lymphocytes into the bloodstream and therefore does not block the interaction of rituximab with B cells, which increases the effectiveness of therapy. It is believed that the ability of rituximab to eliminate B cells is realized through several mechanisms, including complement-dependent and antibody-dependent cellular cytotoxicity, as well as induction of apoptosis [10]. In turn, depletion of B cells can have a significant impact on the basic mechanisms of the development of human autoimmune diseases and has a serious pathogenetic basis: • weakening of the antigen-presenting function of B cells in relation to the induction of proliferation and cytokine synthesis by CD4+ T cells; • destruction of aberrant germ centers, which leads to a decrease in the formation of autoantigen-specific memory B cells, plasma cells and antibody synthesis; • depletion of plasma cell precursors, which leads to suppression of antibody synthesis and formation of immune complexes; • modulation of the activity of other autoreactive cells due to dysfunction of T cells; • activation of T-regulatory cells (CD4+ CD25+). Currently, the possibility of effective control of autoimmune pathological conditions by depletion (and/or modulation of function) of B cells has been proven in clinical studies. This is evidenced by the high effectiveness of rituximab in the most common and severe autoimmune rheumatic disease, rheumatoid arthritis (RA), which served as the basis for registration of the drug for the treatment of this disease. A detailed analysis of the results of clinical trials of rituximab in RA is presented in previous publications [7,11,12]. In recent years, clinical experience with the use of rituximab for the treatment of other human autoimmune diseases has been rapidly accumulating [13–15]. It should be especially emphasized that in most cases, rituximab was prescribed to patients with very severe diseases who were resistant to standard glucocorticoid and cytotoxic therapy, intravenous immunoglobulin, and extracorporeal blood purification methods, often for life-saving reasons. Systemic lupus erythematosus Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease, the pathogenesis of which is based on defects in immunoregulation, leading to uncontrolled hyperproduction of autoantibodies to the components of one's own tissues and the development of chronic inflammation affecting many organs and systems. Moreover, B lymphocytes play a key role in the development of immunopathological processes in this disease [16]. Currently, there is data on the use of rituximab in more than 100 patients with SLE, both adults and children, the results of which are summarized in a review [17]. The results of the studies allow us to draw the following main conclusions: • Overall, treatment with rituximab was associated with a significant reduction in disease activity in more than 80% of patients. • Prescription of rituximab is highly effective in patients with SLE with active extrarenal manifestations of the disease (serositis, polyarthritis, skin lesions, stomatitis, fever, anemia), with progressive lupus nephritis (morphological type III–IV according to the WHO classification). • Rituximab may be the drug of choice in patients with “critical” SLE caused by severe central nervous system damage (coma, convulsions, psychosis), as well as multiorgan thrombosis associated with catastrophic antiphospholipid syndrome. • Preliminary results indicate the high effectiveness of repeated courses of rituximab therapy in the event of an exacerbation. Sjögren's syndrome Sjögren's syndrome (SS) is a systemic autoimmune disease affecting the exocrine glands and manifests itself as persistent dry mouth and eyes associated with dysfunction of the salivary and lacrimal glands [18]. SS is very common in the population, occurring with a frequency of 0.6%–3.3% or 4 new cases per 100,000 population per year. SS most often develops in middle-aged women, the ratio of women to men ranges from 14:1 to 24:1. There are primary Sjogren's syndrome (disease) and secondary SS, which develops in patients with RA and other autoimmune rheumatic and non-rheumatic diseases. SS is a potentially serious disease characterized by the development of a wide range of extraglandular (systemic) manifestations and a high risk of lymphomas, which reflects the fundamental role of B-cell hyperreactivity in the immunopathogenesis of the disease [3]. It is believed that primary SS represents a unique model for studying the processes underlying the transformation of polyclonal B-cell activation into oligo-(mono-) clonal proliferation of B-cells, ultimately leading to the development of malignant B-cell lymphoproliferative diseases. Currently, pathogenetically based therapy for SS has practically not been developed and is limited to symptomatic treatment of keratoconjunctivitis sicca and xerostomia and the use of GCs and cytostatics in patients with severe systemic manifestations of the disease. Attempts to prescribe TNF-a inhibitors have yielded conflicting results and are not sufficiently substantiated pathogenetically. Data regarding the effectiveness of rituximab in SS are summarized in Table 1. The effect of rituximab treatment on the manifestations of keratoconjunctivitis sicca and xerostomia was analyzed in 3 studies. According to JE Gottenberg et al. [20], 3 patients experienced significant improvement, and 2 patients experienced stabilization of clinical symptoms. Similar results were obtained by J. Pijpe et al. [21]. At the same time, R. Seror et al. [22] did not reveal a significant effect of treatment on the manifestations of sicca syndrome. In a study by V. Davauchelle-Pensec et al. [23] established the effectiveness of rituximab in relation to such manifestations of the disease as weakness, dry mouth, joint damage, general condition (SF-36), and functional state of the lungs (1 patient). A particularly pronounced effect was noted in relation to systemic manifestations of the disease. According to JE Gottenberg et al. [20], significant positive dynamics were found in 5 out of 6 patients, which made it possible to significantly reduce the dose of GC. R. Seror et al. [22] demonstrated the high effectiveness of rituximab against systemic manifestations in 9 out of 11 patients. Of undoubted interest are materials concerning the effectiveness of rituximab in patients with SS and lymphomas. In a study by JE Gottenberg et al. [209] complete remission was observed in 1 patient, and according to J. Pijpe et al. [21], complete remission was achieved in 3, and partial – in 2 patients. Disease progression occurred in only 1 patient. According to R. Seror et al. [22], the development of remission occurred in 4 out of 5 patients with lymphoma. In addition, there is evidence of the high effectiveness of rituximab as adjuvant therapy for aggressive B-cell lymphomas. M. Vougarelis et al. [24] presented results of long-term remission of aggressive diffuse B-cell lymphoma in 6 patients with primary SS who received combination therapy with cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) and rituximab. In general, the effectiveness of therapy was higher than in the comparison group in patients receiving only cytotoxic therapy without rituximab. Idiopathic inflammatory myopathies (IIM) Idiopathic inflammatory myopathies (IIM) are a group of autoimmune rheumatic diseases that can occur both as independent nosological forms and as a syndrome in various rheumatic diseases [25]. The most common forms of IIM are polymyositis (PM) and dermatomyositis (DM). The pathogenesis of IIM is based on autoimmune muscle damage, which has its own characteristics in PM and DM. In PM, the cellular infiltrate is dominated by CD 8+ T lymphocytes and macrophages, and in DM, CD 4+ T lymphocytes predominate. The development of IIM can be accompanied by the synthesis of a wide range of autoantibodies, which are called myositis-specific. Treatment of PM/DM remains largely empirical and usually consists of a combination of GCs and immunosuppressive therapy, but treatment is ineffective in many patients. Therefore, the experience of using rituximab in IIM is of undoubted interest (Table 2). As can be seen from the table, treatment with rituximab was effective in the majority of patients, which was manifested in the normalization (or significant increase) of muscle strength and a decrease in CPK concentrations. In patients with DM, relief of skin manifestations was noted in all cases. In patients with antisystetase syndrome, normalization of pulmonary function was noted. Systemic vasculitis Systemic vasculitis associated with the synthesis of antineutrophil cytoplasmic antibodies (ANCA) is a group of systemic autoimmune diseases characterized primarily by small vessel vasculitis and ANCA synthesis [32]. There are 2 main forms of these vasculitis: Wegener's granulomatosis (WG), which is characterized by the formation of granulomas and destructive damage to the upper respiratory tract, and microscopic polyangiitis (MPA), in which these manifestations, as a rule, are not observed. The use of rituximab in ANCA-associated systemic vasculitis is theoretically justified and is determined by the important pathogenetic role of ANCA (antibodies to proteinase 3 and antibodies to myeloperoxidase) in the development of systemic vascular damage. In addition, B cells are involved in the formation of granulomas during hepatitis B, and an increase in their level in the peripheral blood is associated with disease activity. Currently, a very large number of clinical studies have been conducted indicating the effectiveness of rituximab in hepatitis B and MPA (Table 3). In general, a clinically pronounced effect on the main clinical manifestations of the disease was observed in more than 90% of patients. Moreover, more than 80% of patients achieved complete remission, and exacerbation of the disease was well controlled by repeated courses of therapy (on average after 9–12 months). It is noteworthy that exacerbation was associated with normalization of B cell levels and an increase in ANCA titers. However, in many patients, remission remained in the absence of treatment or while taking small doses of GCs, despite the normalization of B-cell levels and an increase in ANCA titers. It should be noted that some manifestations of hepatitis B, in particular retro-orbital granuloma, are less “sensitive” to rituximab than granulomatous pulmonary lesions. In some patients, rituximab was prescribed in combination with other immunosuppressive drugs, including cyclophosphamide, methotrexate, azathioprine and mycophenolate mofetil, while in others it was prescribed as monotherapy in combination with GC alone. It should be noted that the full clinical effect of treatment with rituximab develops by the end of the third month, which dictates the need for further study of optimal combination therapy regimens. Cryoglobulinemic vasculitis Mixed cryoglobulinemia (MC) is a systemic vasculitis associated with the proliferation of B cell clones that synthesize “pathogenic” IgM, which has the activity of rheumatoid factor (RF) [32]. KS leads to the development of a wide range of clinical manifestations, the severity of which varies from moderate cutaneous vasculitis such as purpura, arthralgia and asthenia (KS syndrome) to severe neurological disorders and kidney damage. Currently, the role of the hepatitis C virus (HCV) in the development of KS has been convincingly proven, which is detected in 60–90% of patients, and cryoglobulinemia develops in 36–55% of patients infected with HCV. At the same time, 15–20% of patients with HCV-associated KS develop severe vasculitis, which, in the absence of effective treatment, can lead to death in 15–20% with HCV-associated KS and in 50% with ESC. Treatment for KS is practically undeveloped. In patients with HCV infection, monotherapy with interferon (IFN)-a is not effective enough and is often accompanied by the development of exacerbations. Combination therapy with IFN-a and ribavirin is more effective, with almost 80% of patients developing remission, but some patients experience severe side effects. The use of GC, cyclophosphamide and plasmapheresis in patients resistant to antiviral therapy also has limited effectiveness and is accompanied by severe side effects. Currently, a series of studies have been conducted (57 patients in total), which are summarized in the review by P. Cocoub et al. [39], indicating the high effectiveness of rituximab in LE (Table 4). Two thirds of the patients had LE associated with VGB infection, and the rest had essential mixed cryoglobulinemia (ECC). The main clinical manifestations of LE were skin lesions (84%), arthralgia (61.4%), peripheral neuropathy (54.4%), glomerulonephritis (31.6%), which were refractory to antiviral therapy (more than half of the patients) and immunosuppressive therapy for the rest. As shown in Table 4, rituximab was effective against the main clinical manifestations of LE, with 80–93% of patients achieving complete (or partial) remission. However, 39% of patients developed an exacerbation after an average of 6.7 months. after the last infusion. At the same time, 8 out of 14 managed to achieve remission after a repeated course of therapy. Notably, rituximab was equally effective in patients with ESC and HCV-related LE. Idiopathic thrombocytopenic purpura Idiopathic thrombocytopenic purpura (ITP) is a common hematological autoimmune disease associated with the synthesis of antiplatelet antibodies, characterized by thrombocytopenia and the risk of bleeding. There are currently 19 studies (313 patients in total) examining the efficacy of rituximab and 29 studies (306 patients) examining the safety of the therapy, the results of which were summarized in a recently published systematic review [40]. The analysis demonstrated the very high effectiveness of rituximab (62.5%) in normalizing platelet concentrations (Table 5). The duration of the effect averaged 10.5 months. Most patients received a standard treatment regimen consisting of 4 weekly infusions of the drug at a dose of 375 mg/m2. Approximately half of the patients had undergone splenectomy before receiving rituximab, which was ineffective. Pemphigus Pemphigus is a potentially fatal autoimmune disease that causes severe damage to the skin and mucous membranes [41]. The development of the disease is based on the synthesis of autoantiber, responding to Desmoglein 1 and 3 and epidermal adhesion molecules, ensuring adhesion between keratinocytes, respectively in the skin and mucous membranes [42]. Recently there were reports of the successful use of rituximab in patients with severe bubbles refractory to standard therapy, including high doses of Civil Code, cytostatics, plasmapheresis and intravenous immunoglobulin [43]. In the study by P. Joly et al. [44], which included 21 patients, treatment with rituximab (375 mg/kg for 4 weeks) led to complete remission in 18 (86%) patients. The duration of remission was an average of 35 months, and in 8 patients it was possible to completely cancel the Civil Code, and in the rest - to significantly reduce the supporting dose of the drug. Similar data were previously obtained by other authors who report a complete remission in 9 out of 11 patients with a fatal course of the disease, but the state of remission was supported by infusion of intravenous immunoglobulin [45]. Piases of the kidneys as already noted, Rituximab has established itself as an effective drug for the treatment of lupus nephritis. Therefore, the results of the use of rituximab in other forms of renal pathology are of particular interest. Membranose nephropathy (MN) is the most common cause (20%) of idiopathic nephrotic syndrome (INS) [46]. Despite the fact that a third of patients may develop spontaneous remission, in almost 40% of cases the process progresses, leading to the development of chronic renal failure within 10 years after the onset of the disease, even despite the ongoing immunosuppressive therapy [47,48]. A characteristic feature of kidney damage in MN is the accumulation of immune deposits on the outside of the basal membrane of the glomerulus of the kidneys. Immune deposits consist of IgG (often IgG4), a membrane -at a compile complex (C5B - C9), as well as poorly described protein molecules, which, as they suggest, can act as autoantigenes. Thus, MN can be a kind of autoimmune disease, the development of which is associated with the synthesis of “jade” autoantibodies. In the process of studying the experimental model of MN, Haymanovsky nephritis, it was shown that one of the probable autoantigen is membrane protein of the podocytes of the kidney, which was called Megalin. Its analogue for MN in a person may have neutral endopeptidase - an enzyme present on the membrane of podocytes and other components of the renal tissue [49]. According to P. Ruggenenti et al. [50], a rituximab treatment is associated with a reliable decrease in proteinuria, an increase in the concentration of serum albumin in patients with INS, resistant to long -term therapy with angiotensin -converting enzymes (APF). The relationship between the effectiveness of therapy with rituximab and the severity of interstitial fibrosis and atrophy of the tubules, according to morphological research in dynamics [51], was noted. In addition, preliminary results indicate the high efficiency of rituximab in children with steroinerezisten nephrotic syndrome [52] and focal segmental glomerulosclerosis, which is often developing after the kidney transplant [53]. These data indicate the prospects for the use of rituximab in nephrology and transplantology [54]. Thus, Rituximab is an extremely effective and relatively safe drug for the treatment of RA and other serious autoimmune diseases. Its implementation in clinical practice can rightfully be considered a major achievement of medicine at the beginning of the 21st century, which has not only important clinical, but also theoretical significance, since it helps to decipher the fundamental links of pathogenesis of autoimmune human diseases. In fact, Rituximab is the ancestor of a new direction in the treatment of autoimmune diseases of a person, which is based on the modulation of the B - cell level of immunity. However, the study of the place of rituximab in clinical medicine is only beginning. Since, as already noted, in most cases the drug was prescribed to patients with a very severe course of diseases, often in life indications, it is not surprising that controlled studies of its effectiveness and safety with most autoimmune diseases (with the exception of RA) have not yet been carried out. Nevertheless, although the above optimistic results are based mainly on the materials of open “pilot” studies or retrospective analysis of therapy outcomes in small groups of patients, they create good prerequisites for the wider implementation of rituximab in clinical practice and should stimulate the organization of large -scale controlled tests necessary To expand official indications for its use.
References 1. Browning JL. B cell move to center stage: novel opportunities for autoimmune disease treatment. Nature Rev. 2006; 5: 564–576 2. Bizzaro N, Tozzoli R, Shoenfeld Y. Are we at stage to predict autoimmune rheumatic diseases? Arthritis Rheum 2007; 56: 1736–1744 3. Hansen A, Lipsky PE, Dorner T. B cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nature Clin Pracr Rheumatol 2007; 3: 561– 4. Edwards JCW, Cambridge G. Rheumatoid arthritis: the predictable effect of small immune complexes in which antibody is also antigen. Br J Rheumatol. 1998; 37:126–130. 5. Youinou P, Jamin C, Saraux A. B-cell: a logical target for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2007; 25: 318–328 6. Driver CB, Ishimori M, Weisman WH. The B cell in systemic lupus erythematosus: a rational target for more effective therapy. Ann Rheum Dis 2007; August 24 on line 7. Edwards JCW, Cambridge G, Leandro MJ. B cell depletion therapy in rheumatic disease Best Pract Res Clin Rheumatol 2006; 20: 915–928 8. Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human antibody to CD20. Blood. 1994; 83:435–445. 9. Boye J, Elter T, Engert A. An overview of the current clinical use of the anti–CD20 monoclonal antibody rituximab. Ann Oncol 2003; 14: 520–535EEEE 10. Johnson P, Glennie M. The mechanism of action of rituximab in the elimination of tumor cells. Semin Oncol 2003 (Supp 2); 30:3–8. 11. Nasonov EL. Prospects for the use of monoclonal antibodies to B lymphocytes (rituximab) in rheumatoid arthritis. Wedge. Pharmacol. Therapy 2006; 1–5:55–58 12. Nasonov E.L. New directions in the treatment of rheumatoid arthritis: prospects for the use of monoclonal antibodies to B lymphocytes (rituximab). RMJ 2006; 25: 1778–1782 13. Solovyov S.K., Kotovskaya M.A., Nasonov E.L. Rituximab in the treatment of systemic lupus erythematosus. RMJ 2005; 13: 1731–1735 14. Nielsen CH, Fassi DE, Hasselbalch HC, et al. B cell depletion with rituximab in the treatment of autoimmune disorders. Expert Opin Biol Ther 2007; 7: 1061–1078 15. Schmidt E, Hunzelman N, Zillikens D, et al. Rituximab in refractory autoimmune diseases Clin Exp Dermatol 2006; 31: 503–508 16. Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001; 2:764–766. 17. Solovyov SK, Torgashina A, Aseeva E, Nasonov E.L. Rituximab. Anti-B cell therapy for systemic lupus erythematosus. Institute of Rheumatology RAMS, Moscow, 2007, 20 p. 18. Moutsopoulos HM, Chused TM, Mann DL, et al. Sjogrens`s syndrome (sicca syndrome): current issues. Ann Intern Med 1980; 92: 212–226 19. Ramos-Casals M, Brito-Zeron P. Emerging biological therapy in primary Sjogren's syndrome. Rheumatology 2007, June 22, on line. 20. Gottenberg J–E, Guillevin L, Lambotte O, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune disease. Ann Rheum Dis 2005;64:913–920. 21. Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjogren`s syndrome: an open–label phase II study. Arthritis Rheum 2005; 64: 913–920 22. Seror R, Sorbet C, Guilleven L, et al. Tolerance and efficacy of rituximab and changed in serum biomarkers in patients with systemic complications of primary Sjogren`s syndrome. Ann Rheum Dis 2006; Sept 1 on lime 23. Davauchelle–Pensec V, Pennec Y, Morvan J, et al. Improvement of Sjogren`s syndrome after two infusions of rituximab (Anti–CD20). Arthritis Care Res 2007; 57: 310–317 24. Voulgarelis M, Giannouli S, Anagnostou D, Tzioufas AG, Combined therapy with rituximab plus cyclophosphamide/doxorubicin/vincristin/prednisolone (CHOP) for Sjogren's syndrome associated B-cell aggressive B cell aggressive non-Hodgkin `s lymphoma. Rheumatology (Oxford) 2004; 43: 1050–1053 25. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003; 362:971–982. 26. Levin TD Rituximab in the treatment of dermatomyositis. Arthritis Rheum 2005; 52:601–607. 27. Noss EH, Hausner–Sypek DL, Weinblatt ME. Rituximab as the therapy for refractory polymyositis and dermatomyositis. J Rheumatol 2006; 33: 1021–1026. 28. Lambotte O, R Kotb, G Maigne et al. Efficacy of Rituximab in refractory polymyositis.J Rheumatology 2005;32:1369–70. 29. Brulhart L, Waldburger J–M, Gabay C. Rituximab in the treatment of antisynthetase syndrome. Ann Rheum Dis 2006; 65: 974–975 30. Cooper MA, Willingham DL, Brown DE, et al. Rituximab for the treatment of juvenile dermatomyositis. Arthritis Rheum 2007; 56: 3107–3111 31. Mok CC, Ho LY, To CH. Rituximab for refractory polymyositis: an open–label prospective study. J Rheumatol 2007; 34: 1864–1868 32. Nasonov E.L., Baranov A.A., Shilkina N.P. Vasculitis and vasculopathy. Yaroslavl. Volga Publishing House, 1999, 612 pp. 33. Flossman O, Jones RB, Jayne DRW, Luqmani RA. Should rituximab be used to treat antineutrophil cytoplasmic antibody associated vasculitis? Ann Rheum Dis 2006;15 June on line. 34. Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener's granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med 2006;173:180–7 35. Aries PM, Hellmich B, Voswinkel J, Both M, Nolle B, Holl–Ulrich K, et al. Lack of efficacy of rituximab in Wegener's granulomatosis with refractory granulomatous manifestations. Ann Rheum Dis 2006;65:853–8. 36. Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum 2005;52:262–8 37. Eriksson P. Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med 2005;257:540–8. 38. Omdal R, Wildhagen K, Hansen T, Gunnarsson R, Kristoffersen G. Anti-CD20 therapy of treatment-resistant Wegener's granulomatosis: favorable but temporary response. Scand J Rheumatol 2005;34:229–32 39. Cocoub P, Delluc A, Saadoun D, et al. Anti–CD20 monoclonal antibody (rituximab) treatment for cryoglobulinemia vasculitis? Where do we stand? Ann Rheum Dis 2007; 20 June on line. 40. Arnold DA, Dentali F, Crowther MA, et al. Systemic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007; 146: 25–33 41. Anhalt GJ, Diaz LA. Research advances in pemphigus. JAMA 2001; 285:652–654 42. Kottke MD, Delva E, Kowalczyk. The desmosome; cell science lessons from human diseases. J Cell Sci 2006; 119: 797–806 43. Schmidt E, Hunzelman N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol 2006; 31: 503–508 44. Joly P, Mouquet H, Roujeau J–C, et al. A single cycle of rituximab for the treatment of severe pemphigus. New Engl J Med 2007; 357: 545–552 45. Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. New Engl J Med 2006; 355: 1772–1779 46. Glassoks RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol 2003; 23: 324–332. 47. Glassock RJ. The treatment of idiopathic membranous nephropathy; a dilemma or a conundrum? Am J Kidney Dis 2004; 44:562–566 48. Perna A, Schieppati A, Zamora J, et al. Immunosuppressive treatment for idiopathic membranous nephropathy: a systemic review. Am J Kidney Dis 2004; 44: 385–401 49. Ronco P, Debiec H. Molecular dissection of target antigens and nephritogenic antibodies in membranous nephropathy; towards epitope-driven therapies. J Am Soc Nephrol 2006; 17: 1772–1774 50. Ruggenenti P, Chiurchiu C, Brusegan V, et al. Rituximab in idiopathic membranous nephropathy; a one-year prospective study. J Amer Soc Nephrol 2003;14:1851–1857 51. Ruggenenti P, Chiurchiu C, Abbate M, et al. Rituximab for idiopathic membranous nephropathy; who can benefit? Clin J Am Soc Nephrol 2006; 1: 738–748 52. Bagga A, Sinha A, Moudgil A. Rituximab in patients with steroid-resistant nephrotic syndrome. New Engl J Med 2007; 356: 2751–2752 53. Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med 2006; 35: 1961–1963 54. Salama AD, Pusey CD. Rituximab in renal disease and transplantation Nature Clin Pract Nephrol 2005; 2:221–230.
Side effects
Any antitumor treatment is fraught with complications, and rituximab is no exception; the range of complications is quite wide, some adverse reactions are common, and some are not avoided by most patients. With combination chemotherapy, the toxicity of rituximab is superimposed by adverse reactions from other drugs in the regimen.
Very often during treatment with rituximab the following develops:
- local swelling of the skin - angioedema, when the area rises like a “cushion” next to completely unchanged tissues;
- the development of infection is associated with the mechanism of action and the main strength of the drug - the destruction of lymphocytes;
- various rashes with itching;
- headaches and often with fever;
- nausea.
After the course, blood test results and G-immunoglobulin levels decrease, but grade 4 drop in level is observed in hardly 1-2%. The decrease in the number of B-lymphocytes is not fatal, but is very long-lasting - up to a year.
With very high sensitivity to rituximab and massive destruction of tumor nodes, the development of tumor lysis syndrome is possible, which must be predicted in advance and preventive measures taken.
Experience with the use of rituximab in the treatment of rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joints, which primarily affects the synovium, as well as articular cartilage and marginal areas of the bone. RA is characterized by a steadily progressive, relapsing course and a sharp decline in quality of life with early disability.
The pathogenesis of RA is complex and not fully understood: it is known that provoking factors in the development of the disease are the activation and proliferation of immunocompetent cells (macrophages, T- and B-lymphocytes), the release of cytokines, growth factors, as well as the synthesis of autoantibodies (for example, anti-citrullinated antibodies) and the formation of immune complexes (rheumatoid factor).
To treat RA, a wide range of drugs are used that have a symptomatic effect or affect individual parts of the pathogenesis of the disease (disease-modifying therapy). Early initiation and duration of basic therapy are considered to be favorable signs of prognosis in RA. The rational use of these drugs in the early stages of the disease significantly improves the immediate, long-term functional and even life prognosis, but in many cases their use does not control the progression of the disease, the development of life-threatening complications, or is associated with severe side effects.
In recent years, the view of RA as a potentially incurable and prognostically unfavorable disease has been revised. This is largely due to the expansion of opportunities for early diagnosis of RA, which makes it possible to begin active therapy at the onset of the disease, and the development of a new class of basic anti-inflammatory drugs (DMARDs), the so-called “biological agents”, which selectively block important stages of immunopathogenesis.
Until recently, the pathogenetic mechanisms of RA development were considered mainly from the point of view of defects in T-cell immunoregulation and overproduction of “pro-inflammatory” cytokines, primarily tumor necrosis factor-a (TNF-α), as well as interleukins (IL-1 and IL-6 ). This served as the basis for the widespread introduction into clinical practice of TNF-α inhibitors, which include chimeric (infliximab) and fully human (adalimumab) antibodies to TNF-α.
However, there is evidence that approximately a quarter of patients with RA do not respond to therapy with TNF-a inhibitors. Some patients, more often than not, after 5-6 injections, and sometimes even at later stages of infliximab therapy, do not notice further clinical improvement (the “escape” effect).
But, as is known, RA is a very heterogeneous disease from the point of view of pathogenetic mechanisms, and hyperproduction of TNF-α is, although the most important, but far from the only mechanism of inflammation and tissue destruction in RA.
Until recently, it was believed that antibody-producing B cells play a secondary role in RA, producing IgM, IgG, and IgA rheumatoid factor (RF). Today, the role of B cells as antigen-presenting cells is undeniable. Differentially capturing the antigen using immunoglobulin on the cell surface, the B cell presents it to the T lymphocyte. B cells that synthesize RF have a unique ability to interact with immune complexes and “present” a wide range of autoantigens, and activated B cells express costimulatory molecules (B7 and CD40) necessary for full activation of T cells. The effector role of B cells in the development of joint destruction in RA is also discussed, which is realized through the synthesis of “pro-inflammatory” cytokines (TNF-α, IL-1 and lymphotoxin), as well as IL-6 and IL-10, which have an additional stimulating effect on B lymphocytes.
The use of anti-B-cell drugs is considered a fairly new method of treating RA - namely, the only drug of this group registered to date - rituximab. Rituximab is a genetically engineered chimeric high-affinity monoclonal antibody to the surface receptor of B lymphocytes - CD20. CD20 is a cell membrane antigen, the expression of which is characteristic of “early” and mature B lymphocytes, but is not expressed on stem, “early” pre-B and plasma cells. Therefore, deletion of CD20 B cells does not impair the B cell immune response. Rituximab is believed to act by removing B cells through a combination of several mechanisms—complement-dependent cellular cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of B-cell apoptosis.
The official indication for rituximab is active RA in adults in combination with methotrexate in the setting of intolerance to or inadequate response to current regimens containing one or more tumor necrosis factor-alpha (TNF-a) inhibitors.
This study is devoted to the use of this drug as part of the creation of a national register of the Republic of Tatarstan.
Materials and methods
The study included six patients whose diagnosis of RA was confirmed according to the 1987 American College of Rheumatology (ACR) criteria. The characteristics of the patients are presented in the table.
All patients received basic therapy for RA: methotrexate at a dose of 7.5–10 mg per week; three patients, in addition, received 7.5–10 mg of glucocorticosteroids per day. All patients also received nonsteroidal anti-inflammatory drugs (NSAIDs) in adequate daily doses. However, despite the therapy, consistently high disease activity was observed. One patient received infliximab infusions, which also had no effect - after the seventh infusion there was about. After stopping infliximab, it was decided to prescribe rituximab. All patients during treatment with rituximab continued to receive basic therapy with methotrexate.
All patients had extra-articular manifestations (rheumatoid nodules - 1, polyneuropathy - 2, keratoconjunctivitis sicca - 1, weight loss and low-grade fever - 4, Raynaud's syndrome - 1 patient).
Rituximab was administered twice with an interval of two weeks: 1000 mg in 500 ml of physiological solution intravenously over 6 hours. Treatment was carried out according to the standard regimen.
Evaluation of clinical and laboratory indicators of the therapeutic effect was carried out before the start of rituximab administration, before the second administration of the drug and 8, 16, 24 and 48 weeks after the first infusion.
The effectiveness of treatment was assessed using the clinical criteria of ACR and the DAS 28 disease activity index (EULAR).
results
All 6 patients included in the registry received 2 infusions of rituximab and were followed for 24–48 weeks.
A positive effect of rituximab therapy was noted in all patients. By the 8th week of treatment, a significant improvement occurred, reaching a maximum by the 16th week. There was a decrease in pain on a visual analogue scale (VAS, mm), duration of morning stiffness, number of painful and swollen joints, and a decrease in the need for NSAIDs until complete withdrawal (p < 0.05). Two patients completely stopped taking glucocorticosteroids, one patient reduced the dose to 5 mg per day. A significant (p < 0.05) decrease in ESR and C-reactive protein was revealed. The effectiveness of therapy according to ACR criteria was 50% in 3 and 70% in 3 patients; according to DAS 28, the effect was considered satisfactory in two, good in three patients; one patient achieved complete clinical and laboratory remission by week 16.
By the 24th week, in two patients the effect weakened, which was reflected in the deterioration of clinical and laboratory parameters. In this regard, a second course of drug therapy was carried out - two infusions with an interval of 2 weeks. By the 32nd week of observation, one patient showed a significant improvement in clinical and laboratory data, the second patient noted the lack of effect after the second course of treatment (the observation period was 4 weeks).
We observed no adverse events during rituximab therapy. The drug's tolerability is assessed as good.
Thus, the use of rituximab in patients with RA, including those with an inadequate response to basic drugs, has a pronounced long-term clinical and laboratory effect. Such therapy can reduce the risk of early disability, improve the quality of life of patients and the long-term prognosis of the disease.
Literature
- Nasonov E. L. New directions in the treatment of rheumatoid arthritis: prospects for the use of monoclonal antibodies to B-lymphocytes (rituximab) // Russian Medical Journal. 2006. 25; 1778–1782.
- Nasonov E. L. Pharmacotherapy of rheumatoid arthritis - a look into the 21st century. Klin: Medicine. 2005. 6: 8–12.
- Nasonov E. L. Treatment of rheumatoid arthritis: current state of the problem // Breast cancer. 2006. 14 (8); 573–577.
- Browning JL B cell move to center stage: novel opportunities for autoimmune disease treatment // Nature Rev. 2006. 5: 564–576.
- Bizzaro N., Tozzoli R., Shoenfeld Y. Are we at stage to predict autoimmune rheumatic diseases? // Arthritis Rheum. 2007. 56: 1736–1744.
- Hansen A., Lipsky PE, Dorner T. B cell lymphoproliferation in chronic inflammatory rheumatic diseases // Nature Clin Pracr Rheumatol. 2007; 3:561.
- Youinou P., Jamin C., Saraux A. B-cell: a logical target for the treatment of rheumatoid arthritis // Clin Exp Rheumatol. 2007. 25: 318–328.
- Edwards JCW, Cambridge G., Leandro MJ B cell depletion therapy in rheumatic disease // Best Pract Res Clin Rheumatol. 2006. 20: 915–928.
I. G. Salikhov , Doctor of Medical Sciences, Professor L. I. Myasoutova M. Yu. Badeeva E. R. Kirillova , Candidate of Medical Sciences S. A. Lapshina , Candidate of Medical Sciences R. D. Abdrakipov KSMU , Kazan
special instructions
Every second person during a drip, especially during the first injections, develops an unpleasant condition - an infusion reaction, when the patient feels a sudden surge of weakness to the point of “shaking”, with nausea and headache, the skin begins to itch in some places and a rash can quickly appear, causing It is difficult to breathe due to spastic contractions of the respiratory tract.
If, despite the preliminary administration of drugs - premedication, a severe infusion reaction has developed, then the administration of rituximab is stopped for a while, additional auxiliary drugs are administered intravenously, and when the condition normalizes, the drip administration of rituximab is continued, but the rate of droplet fall is halved.
As a rule, the intensity of the reaction decreases by the next dropper, and 99% of patients tolerate the last 8th dropper without a reaction. However, before administering rituximab, drugs are always administered prophylactically to prevent allergic manifestations, pain and fever.
Rituximab
The incidence of side effects is classified according to the recommendations of the World Health Organization: very often (≥ 1/10); often (≥1/100 and <1/10); uncommon (≥ 1/1000 and < 1/100); rare (≥1/10000 and <1/1000); very rare (<1/10000), frequency unknown (cannot be calculated based on available data).
The drug Rituximab in the treatment of low-grade or follicular non-Hodgkin lymphoma - monothepapia / maintenance therapy
Adverse reactions were reported up to 12 months after monotherapy and up to 1 month after maintenance therapy with Rituximab.
Infectious and parasitic diseases: very often - bacterial and viral infections; often - respiratory tract infections*, pneumonia*, sepsis, herpes zoster*, infections accompanied by fever*, fungal infections, infections of unknown etiology.
Disorders of the blood and lymphatic system: very often - leukopenia, neutropenia; often - thrombocytopenia, anemia; uncommon - lymphadenopathy, bleeding disorder, transient aplastic anemia, hemolytic anemia.
Disorders of the respiratory system, chest and mediastinal organs: often - rhinitis, bronchospasm, cough, respiratory diseases, shortness of breath, chest pain; uncommon - hypoxia, impaired pulmonary function, bronchiolitis obliterans, bronchial asthma.
Immune system disorders: very often - angioedema; often - hypersensitivity reactions.
Metabolic and nutritional disorders: often - hyperglycemia, weight loss, peripheral edema, facial edema, increased LDH activity, hypocalcemia.
General disorders and disorders at the injection site: very often - headache, fever, chills, asthenia; often - pain in tumor foci, flu-like syndrome, hot flashes, weakness; Uncommon: pain at the injection site.
Gastrointestinal disorders: very often - nausea; often - vomiting, diarrhea, dyspepsia, lack of appetite, dysphagia, stomatitis, constipation, abdominal pain, sore throat; infrequently - abdominal enlargement.
Disorders of the cardiovascular system: often - decreased blood pressure, increased blood pressure, orthostatic hypotension, tachycardia, arrhythmia, atrial fibrillation*, myocardial infarction*, cardiac pathology*; uncommon - left ventricular heart failure*, ventricular and supraventricular tachycardia*, bradycardia, myocardial ischemia*, angina*.
Nervous system disorders: often - dizziness, paresthesia, hypoesthesia, sleep disturbance, anxiety, agitation, vasodilation; infrequently - perversion of taste.
Mental disorders: infrequently - nervousness, depression.
Musculoskeletal and connective tissue disorders: often - myalgia, arthralgia, muscle hypertonicity, back pain, neck pain, pain.
Disorders of the skin and subcutaneous tissues: very often - itching, rash; often - urticaria, increased sweating at night, sweating, alopecia*.
Visual disturbances: often - lacrimation disorders, conjunctivitis.
Hearing and labyrinthine disorders: often - pain and tinnitus. Laboratory and instrumental data: very often - a decrease in the level of immunoglobulins G (IgG).
* - frequency is indicated only for adverse reactions ≥ grade 3 in accordance with the National Cancer Institute toxicity criteria (NCI-CTC).
The drug Rituximab in combination with chemotherapy (R-CHOP, R-CVP, R-FO for non-Hodgkin's lymphoma and chronic lymphocytic leukemia
The following are severe adverse reactions in addition to those observed with monotherapy/maintenance therapy and/or occurring at a higher frequency.
Infectious and parasitic diseases: very often - bronchitis; often - acute bronchitis, sinusitis, hepatitis B* (reactivation of the hepatitis B virus and primary infection).
Disorders of the blood and lymphatic system: very often - neutropenia**, febrile neutropenia, thrombocytopenia; often - pancytopenia, granulocytopenia.
Disorders of the skin and subcutaneous tissues: very often - alopecia; often - skin diseases.
General disorders and disorders at the injection site: often - fatigue, chills.
* — frequency is indicated based on observations during the treatment of relapsing/chemoresistant chronic lymphocytic leukemia according to the R-FC regimen.
** - prolonged and/or delayed neutropenia was observed after completion of R-FC therapy in previously untreated patients or in patients with relapsed/chemoresistant chronic lymphocytic leukemia.
The following are adverse events occurring during rituximab therapy with equal frequency (or less frequently) compared to the control group: hematotoxicity, neutropenic infections, urinary tract infections, septic shock, pulmonary superinfections, implant infections, staphylococcal septicemia, pulmonary infection, mucous discharge from nose, pulmonary edema, heart failure, sensory disturbances, venous thrombosis, incl. deep vein thrombosis of the extremities, mucositis, influenza-like illness, edema of the lower extremities, decreased left ventricular ejection fraction, increased temperature, deterioration of general health, fall, multiple organ failure, bacteremia, decompensation of diabetes mellitus. The safety profile of Rituximab in combination with MCP, CHVP-IFN chemotherapy is no different from that of Rituximab in combination with CVP, CHOP or FC in appropriate populations.
Infusion reactions
Monotherapy with Rituximab (for 4 weeks)
More than 50% of patients experienced events resembling infusion reactions, most often during the first infusions. Infusion reactions include chills, trembling, weakness, shortness of breath, nausea, rash, hot flashes, low blood pressure, fever, itching, urticaria, tongue irritation or swelling of the larynx (angioedema), rhinitis, vomiting, tumor pain, headache, bronchospasm . The development of signs of tumor lysis syndrome has been reported.
The drug Rituximab in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin's lymphoma: R-CHOP for diffuse large B-cell non-Hodgkin's lymphoma: R-FC for chronic lymphocytic leukemia
Grade 3 and 4 infusion reactions during infusion or within 24 hours after infusion of Rituximab were observed during the first cycle of chemotherapy in 12% of patients. The incidence of infusion reactions decreased with each subsequent cycle and by the 8th cycle of chemotherapy, the incidence of infusion reactions decreased to less than 1%. Infusion reactions, in addition to those mentioned above (with monotherapy with Rituximab), included: dyspepsia, rash, increased blood pressure, tachycardia, signs of tumor lysis syndrome, in some cases - myocardial infarction, atrial fibrillation, pulmonary edema and acute reversible thrombocytopenia.
Infections
Monotherapy with Rituximab (for 4 weeks)
Rituximab causes depletion of the B cell pool in 70-80% of patients and a decrease in serum immunoglobulin concentrations in a small number of patients. Bacterial, viral, fungal infections and infections of unspecified etiology (all, regardless of cause) develop in 30.3% of patients. Severe infections (grades 3 and 4), including sepsis, were noted in 3.9% of patients.
Maintenance therapy (non-Hodgkin's lymphoma) up to 2 years
During therapy with Rituximab, an increase in the overall incidence of infections was observed, including grade 3-4 infections. There was no increase in the incidence of infectious complications with maintenance therapy lasting 2 years. Fatal progressive multifocal leukoencephalopathy (PML) has been reported in patients with non-Hodgkin's lymphoma after disease progression and re-treatment.
The drug Rituximab in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin's lymphoma; R-CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
There was no increase in the incidence of infections or infestations when treated with Rituximab according to the R-CVP regimen. Upper respiratory tract infections were the most common (12.3% in the R-CVP group). Serious infections occurred in 4.3% of patients receiving R-CVP chemotherapy; No life-threatening infections were reported. The proportion of patients with grade 2-4 infections and/or febrile neutropenia in the R-CHOP group was 55.4%. The overall incidence of grade 2–4 infections in the R-CHOP group was 45.5%. The incidence of grade 2-4 fungal infections in the R-CHOP group was higher than in the CHOP group due to a higher incidence of local candidiasis and amounted to 4.5%. The frequency of herpetic infections of grade 2-4 severity was higher than in the CHOP group due to a higher frequency of local candidiasis and amounted to 4.5%. The incidence of grade 2-4 herpes infection was higher in the R-CHOP group than in the CHOP group and was 4.5%. In patients with chronic lymphocytic leukemia, the incidence of hepatitis B (hepatitis B virus reactivation and primary infection) of grade 3-4 in the R-FC group was 2%.
From the blood system
Monotherapy with Rituximab (for 4 weeks)
Severe thrombocytopenia (grades 3 and 4) was observed in 1.7% of patients, severe neutropenia in 4.2% of patients, and severe anemia (grades 3 and 4) in 1.1% of patients.
Maintenance therapy (non-Hodgkin's lymphoma) up to 2 years
Leukopenia (grades 3 and 4) was observed in 5% of patients, and neutropenia (grades 3 and 4) in 10% of patients receiving Rituximab. The incidence of thrombocytopenia (grade 3-4 severity) was low and amounted to <1%. Approximately 50% of patients for whom B-cell recovery data were available took 12 months or more to recover B-cell counts to normal levels after completion of induction therapy with Rituximab.
The drug Rituximab in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin's lymphoma; R-CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
Severe neutropenia and leukopenia: in patients receiving Rituximab in combination with chemotherapy, grade 3 and 4 leukopenia were observed more often than in patients receiving chemotherapy alone. The incidence of severe leukopenia was 88% in patients receiving R-CHOP and 23% in patients receiving R-FC. The incidence of severe neutropenia was 24% in the R-CVP group, 97% in the R-CHOP group, and 30% in the R-FC group in previously untreated chronic lymphocytic leukemia. The higher incidence of neutropenia in patients receiving Rituximab and chemotherapy was not associated with an increased incidence of infections and infestations compared with patients receiving chemotherapy alone. In patients with recurrent or chemoresistant chronic lymphocytic leukemia and in previously untreated patients after treatment with the R-FC regimen, in some cases neutropenia was characterized by a long course and later manifestations.
Severe anemia and thrombocytopenia (grades 3 and 4): there was no significant difference in the incidence of anemia of grades 3 and 4 between groups. In the R-FC group, in the first line of treatment for chronic lymphocytic leukemia, anemia of grades 3 and 4 occurred in 4% of patients, thrombocytopenia of grades 3 and 4 - in 7% of patients. In the R-FC group with recurrent or chemoresistant chronic lymphocytic leukemia, anemia of grades 3 and 4 occurred in 12% of patients, thrombocytopenia of grades 3 and 4 - in 11% of patients.
From the cardiovascular system
Monotherapy with Rituximab (for 4 weeks)
Side effects from the cardiovascular system were noted in 18.8%. The most common occurrences are increases and decreases in blood pressure. In isolated cases, cardiac arrhythmias of grade 3 and 4 were observed (including ventricular and supraventricular tachycardia) and angina pectoris.
Maintenance therapy (non-Holzhkin lymphoma) up to 2 years
The incidence of grade 3 and 4 cardiovascular events was similar in patients receiving Rituximab and those not receiving it. Serious cardiovascular events occurred in less than 1% of patients not receiving Rituximab and in 3% of patients receiving Rituximab (atrial fibrillation in 1%, myocardial infarction in 1%, left ventricular failure in <1%, myocardial ischemia in <1%). %).
The drug Rituximab in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin's lymphoma; R -CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
The incidence of grade 3 and 4 cardiac arrhythmias, mainly supraventricular arrhythmias (tachycardia, atrial flutter and atrial fibrillation), was higher in the R-CHOP group and amounted to 6.9%. All arrhythmias developed either in connection with the infusion of Rituximab or were associated with predisposing conditions such as fever, infection, acute myocardial infarction, or concomitant diseases of the respiratory and cardiovascular systems. The R-CHOP and CHOP groups did not differ in the incidence of other grade 3 and 4 cardiac adverse events, including heart failure, myocardial disease, and manifestation of coronary artery disease.
The overall incidence of grade 3 and 4 cardiovascular events was low both in first-line treatment of chronic lymphocytic leukemia (4% in the R-FC group) and in the treatment of relapsed/chemoresistant chronic lymphocytic leukemia (4% in the R-FC group).
Nervous system
The drug Rituximab in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin's lymphoma; R-CHOP for diffuse large B-cell lymphoma; R-FC for chronic lymphocytic leukemia
Patients (2%) in the R-CHOP group with cardiovascular risk factors developed cerebroembolic events during the first cycle of therapy, in contrast to patients in the CHOP group who developed cerebroembolic events during the observation period without treatment. There was no difference between groups in the incidence of other thromboembolism.
The overall incidence of grade 3 and 4 neurological impairment was low both in the first-line treatment of chronic lymphocytic leukemia (4% in the R-FC group) and in the treatment of relapsed/chemoresistant chronic lymphocytic leukemia (3% in the R-FC group).
IgG concentration
Maintenance therapy (non-Hodgkin's lymphoma) up to 2 years
After induction therapy, IgG concentrations were below the lower limit of normal (<7 g/L) in the Rituximab-treated and untreated groups. In the group not receiving Rituximab, the median IgG level consistently increased and exceeded the lower limit of normal, while the median IgG level did not change in the group receiving Rituximab. In 60% of patients treated with Rituximab for 2 years, IgG concentrations remained below the lower limit. In the group without treatment with Rituximab, after 2 years the IgG concentration remained below the lower limit in 36% of patients.
Special categories of patients
Monotherapy with Rituximab (for 4 weeks)
Elderly age (≥65 years): the frequency and severity of all adverse reactions and grade 3 and 4 adverse reactions does not differ from that in younger patients.
Combination therapy
Older age (65 years and older): In first-line therapy and in treatment of relapsed/chemoresistant chronic lymphocytic leukemia, the incidence of grade 3 and 4 side effects from the blood and lymphatic system was higher compared to younger patients.
High tumor load (diameter of single lesions more than 10 cm): increased frequency of grade 3 and 4 adverse reactions.
Repeated therapy: the frequency and severity of adverse reactions do not differ from those during initial therapy.
Information on the post-registration use of Rituximab for non-Hodgkin's lymphoma and chronic lymphocytic leukemia
From the cardiovascular system: severe cardiovascular events associated with infusion reactions, such as heart failure and myocardial infarction, mainly in patients with a history of cardiovascular disease and/or receiving cytotoxic chemotherapy; very rarely - vasculitis, mainly cutaneous (leukocytoclastic).
From the respiratory system: respiratory failure and pulmonary infiltrates caused by infusion reactions; In addition to pulmonary adverse events due to infusion reactions, interstitial lung disease, in some cases fatal, has been observed.
From the circulatory and lymphatic system: reversible acute thrombocytopenia associated with infusion reactions.
From the skin and its appendages: rarely - severe bullous reactions, toxic epidermal necrolysis and Stevens-Johnson syndrome, in some cases with death.
From the nervous system: rarely - neuropathy of the cranial nerves in combination with peripheral neuropathy or without it (marked decrease in visual acuity, hearing, damage to other sense organs, paresis of the facial nerve) during various periods of therapy up to several months after completion of the course of treatment with the drug Rituximab.
Cases of posterior reversible encephalopathy (PRE8)/posterior reversible leukoencephalopathy syndrome (PRLS) have been reported in patients treated with Rituximab. Symptoms included visual disturbances, headache, seizures and mental disturbances, with or without increased blood pressure. The diagnosis of PRES/PRLS can be confirmed using brain imaging techniques. In the reported cases, patients had risk factors for developing PRES/PRLS, such as underlying disease, hypertension, immunosuppressive therapy and/or chemotherapy.
From the body as a whole, reactions at the injection site: rarely - serum sickness.
Infections: reactivation of hepatitis B virus (in most cases with a combination of rituximab and cytotoxic chemotherapy); as well as other severe viral infections (primary infection, viral reactivation or exacerbation), some of which were fatal. Most patients received rituximab in combination with chemotherapy or in combination with hematopoietic stem cell transplantation. Examples of such severe viral infections are infections caused by herpes viruses (cytomegalovirus, Varicella zoster, Herpes simplex), JC polyomavirus (PML), hepatitis C virus. When prescribing rituximab for indications not covered by the instructions for medical use in patients with previously diagnosed Kaloshi sarcoma progression of sarcoma was observed (most patients were HIV positive).
From the gastrointestinal tract: perforation of the stomach and/or intestines (possibly fatal) when Rituximab is combined with chemotherapy for non-Hodgkin's lymphoma.
From the blood and lymphatic system: rarely - neutropenia that occurred 4 weeks after the last administration of Rituximab; a transient increase in IgM levels in patients with Waldenström's macroglobulinemia, followed by a return to its original value after 4 months.
Interaction
Rituximab is included in many drug combinations that are being studied in clinical trials not only for effectiveness, but also for safety for patients. For the treatment of oncohematological processes, certain standard combinations are recommended, in which the toxicity is minimally identical with the maximum effectiveness of each drug individually. For drip administration of rituximab, special systems are used, since the drug molecules are combined with the material of conventional droppers.
Fertility, pregnancy and lactation
Antitumor drugs are not studied in pregnant and lactating women, as well as in children. In preclinical studies, of course, experiments can be carried out with pregnant animals, but the coincidence of reactions in animals and humans is absolutely not guaranteed.
Immunoglobulins G are able to pass into breast milk and the fetus; in those who accidentally became pregnant during MAB treatment, newborn blood tests showed a decrease in the number of B lymphocytes.
Women of reproductive age are strongly recommended to use contraception during therapy and for at least a year after its completion.