Pharmacological properties of the drug Tibolone
A drug for the treatment of menopausal syndrome that does not cause regular withdrawal bleeding. Stabilizes the functioning of the hypothalamic-pituitary system during menopause, which is achieved due to the estrogenic, progestogenic and weak androgenic properties of tibolone. Tibolone is rapidly metabolized to form three compounds that provide its pharmacotherapeutic effect. Two metabolites, 3α-OH-tibolone and 3β-OH-tibolone, have predominantly estrogenic activity, and the third, the delta-4 isomer, has progestogenic and weak androgenic activity. After oral administration, tibolone is quickly and completely absorbed. Due to rapid metabolism, tibolone plasma levels are very low. Maximum plasma concentrations of tibolone metabolites are achieved 1–1.5 hours after administration. Excretion of tibolone occurs mainly in the form of metabolites. A small amount is excreted in urine, and most is excreted in bile and feces. The half-life of metabolites is approximately 7 hours without subsequent accumulation.
Ladybon®
Ladybon® is not intended for use as a contraceptive and does not protect against unwanted pregnancy. The decision to start taking Ledibon® should be based on an assessment of the benefit/risk ratio, taking into account all individual risk factors, and in women over 60 years of age, the increased risk of stroke should also be taken into account.
For the treatment of postmenopausal symptoms, Ledibon® should be prescribed only for symptoms that adversely affect quality of life. In all cases, it is necessary to conduct a thorough assessment of the risks and benefits of therapy at least once a year, and therapy with Ledibon® should be continued only for a period of time when the benefits of therapy outweigh the risks. It is necessary to carefully assess the risk of stroke, the risk of developing breast cancer and endometrial cancer in each woman with an intact uterus (see section “Side effects”), taking into account all individual risk factors, the incidence and characteristics of both types of cancer and stroke in terms of curability , morbidity and mortality. Evidence of the relative risk associated with hormone replacement therapy (HRT) or tibolone use for the treatment of premature menopause is limited. However, the benefit/risk ratio may be more favorable in women with premature menopause than in older women due to the lower absolute risk in younger women.
Medical examination/observation Before starting or resuming therapy with Ladybon®, an individual and family medical history should be obtained.
Physical examination (including examination of the pelvic organs and mammary glands) should be carried out taking into account medical history, absolute and relative contraindications. During therapy, preventive repeat examinations are recommended, the frequency and nature of which are determined by the individual characteristics of the patient, but at least once every 6 months. In particular, the woman should be informed about the need to inform the doctor about changes in the mammary glands.
Examinations, including appropriate imaging modalities such as mammography, should be performed according to a currently accepted examination regimen, adapted to the clinical needs of each patient, but at least every 6 months. Reasons for immediate discontinuation of therapy and immediate consultation with a doctor Therapy should be discontinued if a contraindication is identified and/or for the following conditions/diseases:
- jaundice or worsening liver function;
- a sudden increase in blood pressure that differs from the patient’s usual blood pressure readings;
- the occurrence of migraine-type headaches.
- Hyperplasia and endometrial cancer
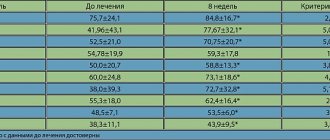
Data from randomized controlled clinical trials are conflicting, but observational studies have shown an increased risk of endometrial hyperplasia or cancer in women taking tibolone (see also section "Side effects"). These studies have shown that the risk of endometrial cancer increases with duration of drug use, and tibolone may increase endometrial thickness as measured by transvaginal ultrasound.
During the first months of treatment, breakthrough bleeding and spotting may occur.
If spotting/bleeding occurs during the use of Ledibon®,
- which last more than 6 months from the start of taking the drug,
- begin 6 months after starting use of the drug Ledibon® and continue even after the patient has stopped using the drug Ledibon®,
You should consult a doctor - this may be a sign of endometrial hyperplasia.
Mammary cancer
Data from various clinical studies from the point of view of evidence-based medicine regarding the risk of developing breast cancer when taking tibolone are contradictory, and further research is required.
Ovarian cancer
Ovarian cancer is much less common than breast cancer.
Long-term (at least 5-10 years) estrogen replacement monotherapy was associated with a slight increase in the risk of developing ovarian cancer.
Some studies, including the Women's Health Initiative study, suggest that long-term HRT combination therapy may have a similar or slightly lower risk.
The Million Women Study showed that the relative risk of ovarian cancer with tibolone was similar to the risk associated with other types of HRT.
Venous thromboembolism HRT preparations containing only estrogens, or combination preparations containing estrogen and a progestogen, may increase the risk of venous thromboembolism (VTE) (i.e. deep vein thrombosis or pulmonary embolism) by 1.3-3 times, in especially during the first year of use (see section “Side effects”).
In an epidemiological study using UK databases, the risk of VTE associated with tibolone was lower than the risk associated with traditional HRT drugs, but due to the fact that only a small proportion of women were taking tibolone at that time, it is not possible exclude a slight increase in risk compared to women who did not take tibolone. Patients with known thrombophilic conditions are at increased risk of developing VTE, and tibolone may increase this risk and is therefore contraindicated in this patient population (see Contraindications).
Risk factors for VTE include estrogen use, older age, major surgery, prolonged immobilization, obesity (body mass index (BMI) >30 kg/m2), pregnancy and the postpartum period, systemic lupus erythematosus and cancer. In patients after surgical interventions, special attention should be paid to preventive measures to prevent VTE in the postoperative period. If prolonged immobilization is necessary after surgery, it is recommended to temporarily stop taking Ledibon® 4-6 weeks before surgery. Treatment should not be resumed until the woman regains motor activity. Women who do not have a history of VTE, but who have first-degree relatives with a history of thrombosis at a young age, may be offered screening (the woman should be informed that screening detects only a subset of thrombophilic conditions). If a thrombophilic condition is identified that is separate from thrombosis in relatives, or a serious disorder (for example, deficiency of antithrombin III, protein S, protein C, or a combination of disorders), taking Ledibon® is contraindicated.
For women already receiving anticoagulant treatment, careful consideration of the benefit/risk of using HRT or tibolone is required.
If VTE develops after starting treatment, the drug should be discontinued. Patients should be advised to seek immediate medical attention if symptoms of potential thromboembolism occur (eg, pain and unilateral swelling of the lower extremity, sudden chest pain, shortness of breath). Coronary heart disease (CHD)
Randomized controlled trials did not provide evidence of protection against myocardial infarction in women with or without coronary artery disease who received HRT with combined drugs (estrogen/progestogen) or drugs containing only estrogen.
Epidemiological studies using the GPRD did not provide evidence of protection against myocardial infarction in postmenopausal women who received tibolone. Ischemic stroke Tibolone therapy increases the risk of ischemic stroke, starting from the first year of use (see section “Side effects”). The absolute risk of stroke is strictly dependent on age, and, therefore, this effect of tibolone is greater with increasing age. If you experience unexplained migraine-like headaches with or without visual disturbances, you should consult a doctor as soon as possible. In this case, you should not take the drug until your doctor confirms that it is safe to continue HRT, since such headaches can be an early diagnostic sign of a possible stroke.
Other states
- Tibolone reportedly resulted in a significant dose-dependent reduction in HDL (high-density lipoprotein) cholesterol (from -16.7% at a dose of 1.25 mg to -21.8% at a dose of 2.5 mg after 2 years of use).
- The total concentration of triglycerides and VLDL also decreased. The decrease in the concentration of total cholesterol and VLDL cholesterol (very low density lipoprotein) was not dose dependent. LDL (low-density lipoprotein) cholesterol concentrations did not change. The clinical significance of these data is not yet known.
- Women with pre-existing hypertriglyceridemia should be closely monitored by a physician during tibolone therapy, as rare cases of significant increases in plasma triglyceride levels, contributing to the development of pancreatitis, have been reported during estrogen therapy for this condition.
- Treatment with tibolone results in a very small decrease in thyroxine-binding globulin (TBG) and total T4. The level of total T3 does not change. Ledibon® reduces the level of sex hormone binding globulin (SHBG), while the levels of corticosteroid binding globulin (CBG) and circulating cortisol do not change.
- The increased risk of dementia should be considered when tibolone therapy is initiated in women over 65 years of age.
- While taking Ladybon®, there is a possibility of fluid retention. In this regard, careful monitoring of patients with cardiac or renal failure is necessary.
Special instructions for the use of Tibolone
Tibolone is not a contraceptive. Tibolone should be prescribed immediately after surgical menopause and no earlier than 1 year after natural menopause (last menstruation). It is not recommended to exceed the recommended daily dose due to the possibility of bleeding from the vagina. While taking tibolone, it is necessary to exercise medical supervision over the condition of patients with migraine, epilepsy, diabetes mellitus, impaired renal function and hypercholesterolemia, as well as a history of these diseases.