Home — For the public
- Map of medical organizations
- Vaccination
- Clinical examination
- Fluorography
- Addresses and opening hours of clinics
- Emergency rooms
- Oncology
- Where to take an HIV test
- Healthy child's office
- Services
- Prevention of CVD
- Disease Prevention
- World Patient Safety Day
- Newspaper "Medical News"
- specialist
- School of Health
— Disease prevention
- HIV infection
- All about vaccination
- All about proper nutrition
- Hepatitis
- Flu
- Dementia
- Schoolchildren's health
- STD
- Tick-borne encephalitis
- Whooping cough
- Measles
- Legionellosis
- Meningococcal infection
- Oncology
- Acute intestinal infection
- Pediculosis
- First aid
- Pneumococcal infection
- Pneumonia
- Prevention of rabies
- Dependency Prevention
- Rotavirus infection
- Diabetes
- Cardiovascular diseases
- Injuries
- Tuberculosis
- Tularemia
- Physical activity
- Obstructive pulmonary disease
- Exotic infections
- Ecology
- Why is swimming in ponds dangerous?
— Hepatitis — Vaccination against hepatitis B
Vaccination against hepatitis B is an effective way to protect not only from the disease itself, but also from its severe consequences. The most significant are cirrhosis and primary liver cancer. The annual mortality rate from them is about 800,000 people, “second only” to such an infectious disease as tuberculosis.
According to WHO experts, in 2022, about 325 million people were registered in the world infected with hepatitis B or C. Unfortunately, a vaccine against hepatitis C has not yet been created, but vaccination against hepatitis B can prevent infection in 98-100% . cases.
Vaccine options
All modern vaccines for the prevention of viral hepatitis B are produced using genetic engineering technology.
A segment of the virus genome is introduced into the genetic material of baker's yeast, which is responsible for the production of the “Australian” (HBsAg) antigen. Vaccines consist of almost 90-95% antigen and only 5-10% of other components. The following vaccinations are used in Russia: “Vaccine against hepatitis B recombinant”, “Regevac V”, “Engerix V”, “Bubo-Kok”, “Bubo-M”, “Shanvak-V”, “Infanrix Hexa”, DPT-GEP B All of these vaccines are weakly reactogenic, interchangeable - that is, the course of vaccinations can be started with one vaccine and finished with another (although it is still preferable to vaccinate within the course with a vaccine from the same manufacturer). They are intended for vaccination of children and adults against hepatitis B.
The second, non-specific, but important component of vaccines is aluminum hydroxide. This substance is a so-called depositing agent in vaccinations and is designed to enhance the immune response. Its purpose is aimed not only at strengthening the immune response, but also at the dosed release of antigen from the site of hepatitis B vaccination. The need for it is dictated by the fact that, as a rule, vaccines based on only one antigen are weakly immunogenic, and in order to achieve the required levels of formed antibodies require either the introduction of more antigen or an increased response to it.
Principles and purposes of vaccination
About 780,000 people die every year from the consequences of hepatitis B. Vaccination is not only the main and important means of preventing viral hepatitis. It may also protect against primary liver cancer. The basis for preventing hepatitis B is the vaccine against this disease. WHO recommends that all infants should receive hepatitis B vaccination as soon as possible after birth, preferably within 24 hours. The dose given at birth should be followed by two or three subsequent doses to complete the vaccination series. In most cases, one of the following two options is considered optimal:
- a three-dose hepatitis B vaccination regimen, in which the first dose (of monovalent vaccine) is given at birth, and the second and third doses (of monovalent or combination vaccine) are given at the same time as the first and third doses of DPT vaccine;
- A four-dose regimen in which the first dose of monovalent vaccine given at birth is followed by 3 doses of monovalent or combination vaccine, usually given along with other vaccines as part of routine childhood immunization, is indicated for children born to mothers infected or with hepatitis B.
After a full series of vaccinations, more than 95% of infants, children of other age groups and young adults develop protective antibody levels. Protection lasts for at least 20 years and possibly a lifetime. All previously unvaccinated children and adolescents under 18 years of age should receive the vaccine if they live in countries with low or moderate endemicity. By the end of 2013, hepatitis B vaccine for infants had been introduced nationally in 183 countries. Global coverage of three doses of hepatitis B vaccine is estimated at 81%, and for Western Pacific countries it reaches 92%.
Is it worth getting vaccinated against hepatitis?
To understand how the hepatitis B vaccine works, it is important to understand what the virus is, how long it can remain viable in the environment, and who is at risk for developing the chronic disease.
Information about the hepatitis B virus
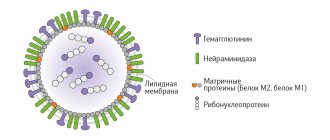
The cause of hepatitis B is a DNA-containing virus, “packaged” in a regular protein shell (capsid) and a lipid membrane – supercapsid. The membranes contain specific proteins HBcAg and HBsAg. Scientists have identified 10 genotypes of the virus, representatives of which are unevenly distributed in different countries of the world.
Penetrating into liver cells, the virus is partially integrated into human DNA. Its fragments remain in hepatocytes for a long time. In most patients, primary infection results in an asymptomatic course (persistence of the virus), and hepatitis can be detected incidentally during a routine blood test.
Prolonged existence of the virus in liver cells can cause primary cancer. Currently, there are 2 hypotheses for its development:
- modification of the cell genome by viral DNA fragments;
- the action of protein X, which triggers the synthesis of virions using host hepatocytes.
In an infected person, the virus can be detected in almost all biological fluids: saliva, tears, blood, as well as in urine and feces. Virions do not penetrate intact skin and mucous membranes.
The virus is highly resistant to the adverse effects of environmental factors: high and low temperatures, freezing and thawing, ultraviolet irradiation, and acids. Outside the human body, it remains viable for 7 days. Inactivation is possible due to boiling, autoclaving, dry heat sterilization in compliance with the temperature and time characteristics of the process.
For whom is the virus most dangerous?
Discussion of the possibility of potential infection, even among people who care about their health, is often negative. Hepatitis B, along with other severe, socially significant infections (HIV, tuberculosis), is one of those diseases that “is not customary to talk about.” The fact of infection stigmatizes patients as individuals who lead an unhealthy lifestyle and are somehow “to blame” for their illness. But is it?
First of all, children are at risk for developing chronic infection. In infants, 70 to 80% of infections are associated with transfusions of blood and its components. WHO statistics are as follows: chronic infection develops in 85-90% of children who become ill within 1 year of life, and in 30-50% of children who become ill between 1 and 6 years. What’s interesting is that an adult who does not have other concomitant pathologies is in a more advantageous position. The probability of developing a chronic infection is no more than 5%.
In areas with a high spread of the virus, a child can be infected from the mother during childbirth, in the family through contact with blood, or through communication among preschool children.
Also at risk for hepatitis B infection are the following categories of people:
- patients with chronic liver diseases (including chronic viral hepatitis C);
- HIV-infected;
- partners of persons infected with hepatitis;
- sexually active people who prefer polygamous relationships;
- patients with diseases that are predominantly sexually transmitted;
- medical personnel working with blood and having contact with biological fluids;
- patients suffering from end-stage kidney disease and receiving hemodialysis;
- social workers involved in caring for patients;
- personnel and persons living in boarding houses, boarding houses;
- men having homosexual contacts;
- people traveling to regions where there is a high incidence of viral hepatitis.
- persons who use injecting drugs.
Vaccine effectiveness
The vaccine is highly safe and effective. More than one billion doses of hepatitis B vaccine have been administered worldwide since 1982. In many countries where typically 8% to 15% of children had chronic hepatitis B virus infection, vaccination has helped reduce rates of chronic infection among immunized children to less than 1% .
After a course of immunization, sufficient immunity is developed in 90% of vaccinated people. Vaccinations can reduce the incidence of hepatitis by 30 times and prevent at least 85-90% of deaths due to this disease. In addition, the risk of getting sick in those born to mothers who are carriers of the infection is reduced by 20 times.
Many researchers call the hepatitis B vaccine the “first cancer vaccine” because it prevents the development of HBV infection, which ultimately leads to hepatocellular carcinoma.
DESCRIPTION OF THE MEDICINE
Hepatitis B recombinant yeast vaccine is a protein sorbed on aluminum hydroxide (HBsAg), synthesized by a recombinant yeast strain and containing antigenic determinants of the surface antigen of the hepatitis B virus.
Appearance: A homogeneous suspension of white, grayish tint, without visible foreign inclusions, which separates when standing into a colorless transparent liquid and a loose precipitate of white with a grayish tint, easily breaking when shaken.
Immunobiological properties
A course of vaccination leads to the formation of specific antibodies to the hepatitis B virus in a protective titer in more than 90% of vaccinated people.
Post-vaccination reactions
Modern vaccinations against hepatitis B are characterized by an extremely high degree of purification, up to 95% of their volume is represented by antigen. In addition, vaccinations consist of only one antigen, the content of which is measured in micrograms. Both of these factors determine that in practice these vaccines are among the safest, “mildest”, and easily tolerated.
The most typical post-vaccination reactions to hepatitis B vaccines are local reactions (ie, those occurring at the injection site). Their frequency is fairly standard for all available vaccines - up to 10% (maximum) of vaccinated people report symptoms such as redness, slight thickening, and discomfort during active movements. The prevalence of local reactions is explained by the action of aluminum hydroxide, a substance that is specifically designed to enhance the inflammatory reaction at the site of drug administration so that as many immunocompetent cells as possible come into contact with the injected antigen.
Much less frequently, with a frequency of about 1% (maximum - 5%), the so-called so-called general reactions, i.e. affecting the body as a whole - a slight increase in body temperature, mild malaise, etc. All of the above reactions are normal (expected), appear within 1-2 days from the moment of vaccination and disappear without treatment within 1-2 days.
SIDE EFFECTS
Side effects with the vaccine are rare. In 1.8-3.0% of cases, slight transient pain, erythema and induration at the injection site are possible, as well as a slight increase in temperature, complaints of malaise, fatigue, joint pain, muscle pain, headache, dizziness, nausea.
These reactions develop mainly after the first two injections and disappear after 2-3 days.
Considering the possibility of the extremely rare development of immediate allergic reactions in particularly sensitive individuals, vaccinated persons must be provided with medical supervision for 30 minutes.
Vaccination sites must be provided with anti-shock therapy.
Contraindications
The only specific and absolute contraindication for vaccines against the hepatitis B virus is an allergy to products containing baker's yeast. Temporary contraindications: severe reaction (temperature above 40oC, swelling, hyperemia > 8 cm in diameter at the injection site) or complication (exacerbation of chronic diseases) to the previous administration of the drug. Routine vaccination is postponed until the end of acute manifestations of the disease or exacerbation of chronic diseases. For mild ARIs, acute intestinal and other diseases, vaccinations can be carried out after the temperature has normalized.
CLAIMS
All cases of increased reactogenicity or development of post-vaccination complications should be reported to:
- Federal Service for Supervision of Consumer Rights Protection and Human Welfare;
- The Ministry of Health and Social Development of Russia and the Federal State Institution GISC named after. L.A. Tarasevich Rospotrebnadzor with subsequent sending of medical documentation: 121002, Moscow, lane. Sivtsev - Vrazhek, 41, tel., fax +7;
- Manufacturer's address: JSC NPK "Combiotech", 117997, Moscow, Miklukho-Maklaya str., 16/10, building 71, tel./fax +7 (495) 330-74-29.