Can anesthesia be replaced with sedation?
The fundamental difference is that in the first case the person is conscious, while in the second he is not.
Treatment during sleep at the Atribeaute Kids clinic is carried out using the safe drug “Sevoran”. During the procedure, the child sleeps soundly under the supervision of the anesthesiology team.
With oxygen sedation, the patient relaxes, the level of stress decreases, but he is conscious, maintains contact with the doctor and assistant, and all reflexes are completely preserved.
The choice of treatment method and sedation is always determined by the doctor based on diagnosis, indications and contraindications.
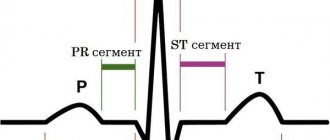
The role of the endothelium in the regulation of vascular tone was first announced in an article by R. Furchott and J. Zawadzki, published in the journal Nature in 1980 [1], which led to the beginning of the study of the properties of nitric oxide (NO), an endothelial relaxation factor, which is a unique biological mediator involved in many physiological and pathophysiological processes. There are three main forms in the body: neutrally charged, relatively stable radical - NO•, nitroxyl anion - NO-, nitrosonium ion - NO+, which have different properties and biological activity [2].
According to its chemical structure, NO is a labile gaseous substance released by endothelial cells during the catabolism of L-arginine with the active participation of NO synthase (NOS) [3]. Synthesized NO stimulates soluble guanylate cyclase, which leads to the formation of cyclic guanosine monophosphate (cGMP), which exerts its effect on target cells. By a negative feedback mechanism, NO affects NOS activity [4].
NOS is a family of hemoprotein enzymes that differ in the amino acid sequence of the protein part of the molecule and the mechanisms that regulate their activity [5]. The main biological role of NOS is the synthesis of NO by cell populations, of which the most studied are the endothelium of blood vessels, neurons and macrophages.
Depending on the cell type, three main isoforms of NOS are distinguished: endothelial (eNOS), neuronal (nNOS) and inducible (iNOS). At the same time, eNOS and nNOS are constantly present in the cytoplasm of cells, and iNOS appears under the influence of certain factors (for example, proinflammatory mediators) [6]. Thus, NO has a number of unique properties: the ability to be synthesized, if necessary, by different cell populations and, along with a paracrine effect, to have a distant effect [7].
NO is critical in regulating cerebral circulation. A decrease in its level in the endothelium of cerebral vessels under the influence of certain factors causes significant changes in their functions and the circulatory system as a whole. For example, a decrease in the effects of endothelium-dependent vasodilators and an increase in vasoconstrictor effects, an increase in blood pressure, disturbances in systemic and regional hemodynamics, an increase in the expression of endothelial adhesive molecules, adhesion and aggregation of platelets to the vascular wall, etc. [8].
The physiological role of NO in the central nervous system is reduced not only to a vasodilating effect, but also to participation in interneuronal synaptic contacts. Possessing neurotransmitter activity, NO is synthesized upon excitation of neurons in response to the influx of Ca2+ ions, then penetrates into neighboring cells, activates the synthesis of cGMP and changes the electrogenesis of neurons [9].
In pathological conditions, such as cerebral ischemia, there is an interaction between the NO and superoxide (O2–) cycles, which are interconnected and develop simultaneously [10]. Describing the interaction of these cycles, it is important to highlight their role in the compensatory and adaptive reactions of the body and emphasize the universal mechanisms of regulation of physiological functions and metabolic processes in cells.
Reactive oxygen species (ROS) and nitrogen species (RNS) are free radicals (superoxide anion (O2•–), hydroxyl radical (OH•), nitric oxide (NO•) and lipid radicals (R•, RO•, ROO•)) [eleven]. APA and their metabolites play a significant role in pathological processes [12]. APA, in addition to NO•, NO–, NO+, includes peroxynitrite (ONOO–), nitrogen dioxide (NO2•), and nitrite anion (NO2–).
NO and its metabolites have pro-oxidant properties [13] and cause peroxidation of phospholipids and mitochondrial membrane proteins, leading to the release of apoptogenic factors into the cytosol. The leading role belongs to ONOO–, which is characterized by the ability to easily penetrate cell membranes, damaging endothelial cells and increasing platelet aggregation. The toxic effect of ONOO– is associated with its concentration: at low levels, the cytoprotective property is realized, at high levels, it is destructive, leading to lipid peroxidation [14].
In contrast to ROS and APA with pro-oxidant effects, the body has an antioxidant biological defense system, the key enzyme of which is superoxide dismutase (SOD), predominantly mitochondrial and cytoplasmic isoforms (Mn-SOD and Cu-Zn-SOD) [15]. In the presence of high concentrations of NO•, SOD can form OHOO–, which can inhibit Cu-Zn-SOD and Mn-SOD. ONOO– can be reduced to NO using nitrite reductases. As a result of nitrite reductase reactions, a decrease in the concentrations of toxic forms of nitrogen is observed [16]. The antioxidant effect of NO is associated with the inhibition of oxidative reactions by Fe3+ ions. At physiological concentrations, NO binds to free and heme Fe2+ ions and inhibits the decomposition of peroxides (Fenton reaction).
Thus, the existing relationships and interdependencies of pro-oxidant and antioxidant systems realize their action with the participation of NO, O2– and their metabolites.
The direct effects of NO predominate in the body under physiological conditions, when NO in low concentrations is synthesized by nNOS and eNOS to implement regulatory and signaling functions. The indirect effect of NO is mediated through reactive forms and manifests itself with an increase in its synthesis associated with the induction of iNOS, which is observed in inflammatory processes of various etiologies and plays an important role in the pathophysiological mechanisms of cerebral ischemia, stroke and other cerebrovascular diseases [17].
The role of NO in the mechanisms of reducing neuronal damage during hypoxia is not clear. In response to hypoxia, the content of nNOS and iNOS increases, and the amount of eNOS in brain vessels decreases due to an increase in the production of ROS [18]. Subsequently, at the early stage of inflammation, under the influence of pro-inflammatory mediators, stimulation of NO production continues with the participation of nNOS. In parallel, the activity of eNOS increases, which causes relaxation of vascular smooth muscle cells and an increase in vascular permeability, promoting fluid exudation. Only NO produced by iNOS contributes to the development of the late phase of inflammation. At this stage of the inflammatory process, NO stimulates the synthesis and release of proinflammatory cytokines, which trigger the migration of macrophages to the site of inflammation [19–22].
The cascade of pathological reactions observed in cerebrovascular insufficiency is diverse and complex, and its implementation is carried out at the molecular and functional levels. A special role belongs to the NO and O2– cycles, the interaction of which determines the direction of reactions. NO, depending on the oxidative metabolism system, can stimulate oxidation, being a powerful pro-oxidant, and have antioxidant activity due to the formation of metabolites. The pro- and antioxidant effects of NO are important in the development of mechanisms of ischemic brain damage.
The pathogenetic mechanisms of cerebrovascular insufficiency are based on the “ischemic cascade” of reactions [23], including a decrease in cerebral blood flow, which leads to an increase in glutamate excitotoxicity, accumulation of Ca2+ and lactic acidosis, which contributes to the activation of proteolysis, the emergence and progression of oxidative stress, the expression of early response genes with the development of depression of plastic proteins and a decrease in energy processes that cause cell death [24–26].
The main role in the formation of pathological reactions in cerebrovascular insufficiency, which develops against the background of atherosclerotic damage to the main arteries of the head and arterial hypertension, belongs to the vascular system. The atherosclerotic process causes changes in cerebral circulation, leads to stenosis and occlusion of the main arteries of the head, impairs the possibilities of collateral redistribution of blood, increases cerebral peripheral vascular resistance, reduces cerebral perfusion, and causes oxygen and energy starvation of neurons. Damage to cerebral vessels in arterial hypertension includes a whole range of disorders: tortuosity and stenosis of the carotid and vertebral arteries, thickening of the intima-media complex, changes in cerebrovascular reactivity [27].
The unifying link in the pathogenesis of atherosclerosis and arterial hypertension is endothelial dysfunction (ED), which is characterized by an imbalance of mediators produced by endothelial cells or manifesting their activity on the surface of the endothelium [28]. ED should be considered as a predictor of the development of cerebrovascular disorders [29].
There is a discrepancy in the terminology of chronic cerebrovascular disease (CVD). In modern literature, the description of this pathology is associated with vascular cognitive disorders [30]. In Russian neurology, CVD is a common diagnosis and, according to statistics, occurs in 75% of cases of all cerebrovascular diseases [31, 32]. Such a high percentage is explained by the “masking” of anxiety and depressive disorders, primary headaches, peripheral vestibulopathy, Alzheimer's disease, symptoms of chronic CVD, which are poorly diagnosed and require a separate approach to treatment.
The search for drugs with endothelial-protective effects has become an urgent problem in clinical pharmacology. Today, it is known that drugs from various pharmacological groups have a positive effect on the functional state of the endothelium, including L-arginine, NO donors, etc. These groups of drugs have not found widespread clinical use and have not proven their effectiveness in clinical practice. To correct impaired endothelial functions, cardiotropic drugs are used, in which the endothelial-protective effect is an important additional, but not the main effect.
Angiotensin-converting enzyme inhibitors (ACE inhibitors).
They have a direct effect on the activity of NOS, thereby neutralizing E.D. They help reduce the level of angiotensin II (an inducer of oxidative stress) and increase the activity of kinins (bradykinin - a stimulator of NO release, prostacyclin). Some representatives of ACE inhibitors (lisinopril) have antioxidant and antithrombotic activity.
AT1 receptor blockers.
With increased production of angiotensin II under conditions of blockade of AT1 receptors, conditions are created for stimulation of AT2 receptors. This may lead to vasodilation and suppression of proliferation through increased NO synthesis and the bradykinin system. AT1 receptor blockers have a beneficial effect on the metabolic profile, including the processes of oxidative stress.
Calcium antagonists.
They have pronounced endothelial protective and antioxidant properties. They have a positive effect on the indicators of various parts of hemostasis.
Thiazide diuretics.
Increases the activity of nNOS and eNOS. They have antioxidant properties and a direct vasodilating effect by increasing the bioavailability of NO and reducing its destruction.
Fibrates.
They reduce the activity of inflammation in the vascular wall and restore the barrier function of the endothelium. They have an antioxidant effect; have a beneficial effect on hemostasis.
statins.
They improve endothelium-dependent vasodilation and reduce the stiffness of the vascular wall.
Antiplatelet agents (acetylsalicylic acid). Its antiplatelet effect is based on the irreversible binding of the platelet cyclooxygenase-1 enzyme with a subsequent decrease in the synthesis of platelet aggregation inducers: prostaglandins and thromboxane A2, which is additionally a powerful vasoconstrictor. Alternative mechanisms of action of acetylsalicylic acid prevent the oxidation of high-density lipoproteins and reduce the formation of free radicals. A reduction in the incidence of cardiovascular complications in patients with arterial hypertension and coronary heart disease has been proven due to the ability of acetylsalicylic acid to restore endothelium-dependent vasodilation.
Apart from cardiovascular drugs with an endothelial protective effect, there are relatively few other effective drugs for the correction of ED in cerebrovascular diseases with proven clinical effectiveness. Based on the important role of the endothelial system in the development of cerebrovascular disorders, on the one hand, and the lack of specific means for the prevention and treatment of ED, on the other, the further search for drugs with an endothelial-protective effect should be considered appropriate and relevant [33].
Drugs with proven endothelial protective activity include divaza, which can additionally solve the problem of vascular cognitive disorders.
Divase contains release-active forms of antibodies to protein S100 (PA AT S100) and eNOS (PA AT eNOS). Receiving R.A. AT is carried out using a fundamentally new technology (US Patent 8,535,664 B2, 2013), which ensures that such forms have a common distinctive property - the ability to have a modifying effect on the original substance (or structurally similar biological molecules) by changing its spatial structure, which entails a change in the physical, chemical and biological properties [34].
The molecular target of the RA AT S100 is the S100 protein, which is involved in the regulation of various intracellular processes: in the transmission of intracellular signals, growth, differentiation, apoptosis of neurons and glia, maintaining the energy metabolism of cells [35, 36], and also in the brain connecting synaptic (information ) and metabolic processes. Influence of R.A. AT S100 promotes a more stable binding of the S100 protein to Ca2+, which triggers a current of Na+ into the cell and Ca2+ outward [37]. The change in transmembrane potential generates an action potential, causing the nerve impulse to propagate to neighboring neurons. The modulating effect of RA AT S100 on the synaptic transmission of various receptors, including GABA, serotonin, σ1-, and NMDA, has been demonstrated. RA AT S100, having a GABA-mimetic and neurotrophic effect, increases the activity of stress-limiting systems and promotes the restoration of neuronal plasticity processes. A wide spectrum of pharmacological activity of RA AT S100, including nootropic, neuroprotective, and membrane stabilizing, has been confirmed in experimental studies [37–41].
The mechanism of action of the second component of the drug Divasa - RA AT eNOS is aimed at restoring endothelial functions and consists in regulating the NO synthase → NO-guanylate cyclase → cGMP cascade. In experimental studies studying the mechanisms of action of the RA AT eNOS, an increase in the activity of eNOS, the production of cGMP, and NO derivatives was noted when taking the drug [39]. A decrease in the ED coefficient to the level of intact animals was also revealed [42]. The data obtained are consistent with data from clinical studies in which an increase in cGMP production and NO production was observed [43] in patients with ED who took the RA AT eNOS. Preclinical studies of the complex drug [44, 45] have proven that divaza prevents the development of pathological consequences of hypoxia in brain tissue and has a pronounced antioxidant and endothelial-protective effect.
In clinical studies [45-48], after 12 weeks of taking Divase in patients with CCVD, there was a significant regression of the main symptoms of the disease (cognitive impairment, weakness, headache, anxiety, sleep disturbances) and normalization of elevated levels of ED markers, such as C-reactive protein , chemoattractant protein-1, endothelin-1, the level of circulating desquamated endothelial cells, etc., which reflects the pathogenetic effect of divase.
It should be noted that taking Divaza was characterized by the absence of adverse drug events; While taking the drug, there were no signs of sedative and muscle relaxant effects, as well as manifestations of addiction or addiction to the drug [46-48]. There are isolated reports on the effectiveness of divaza in transient cerebrovascular accidents. Taking the drug allowed for more effective treatment and minimized the risk of developing a vascular accident. During treatment with Divasa, an improvement in blood supply to the affected area of the brain was noted [49].
In clinical practice, there has also been an improvement in cognitive functions, emotional state and quality of life in patients with cerebrovascular diseases associated with arterial hypertension and/or cerebral atherosclerosis taking Divaza as part of complex therapy [50].
There is a positive experience of taking divaza by patients in the early recovery period of ischemic stroke as a nootropic, vasoactive, antioxidant and endothelium-protective drug. The most significant results were observed in the correction of cognitive and emotional disorders. At the same time, a significant improvement in cognitive functions, including memory, developed within a month of taking the drug. A pronounced response to therapy was observed by the 3rd month of treatment [51].
The experience gained in clinical use and the results of experimental studies of Divase allow us to consider the use of the drug as a promising strategy in the treatment of cerebrovascular disorders, which restores impaired endothelial function and improves cognitive functions in patients with mild and moderate vascular cognitive disorders. It should be noted that the effect of the drug is due to its influence on various parts of the pathological process, including the pro- and antioxidant system, as well as endothelial dysfunction. Proven clinical effectiveness and the absence of adverse events allow us to recommend Divaza for widespread use in patients with cerebrovascular pathology, even with chronic forms.
The authors declare no conflict of interest.
When will nitrous oxide treatment be effective?
1. Age over 3 years. Children are not psychologically ready for this type of manipulation and may be scared.
2. The child sits in the dental chair by himself - without panic fear or hysteria, but at the same time experiences slight discomfort. In this case, the oxygen mixture will help you relax and take your mind off unpleasant sensations.
3. Nasal breathing. The mask should remain on the face throughout the entire procedure, tightly covering the nose. This is due to the fact that the effect of the gas occurs gradually. First, the patient breathes pure oxygen, then the anesthesiologist gradually changes the gas ratio, increasing the nitrogen concentration. If you periodically remove or adjust the mask, the desired effect will not be achieved.
Overdose
An overdose of Nitrous Oxide can be manifested by the following symptoms: respiratory depression, acute hypoxia, decreased blood pressure, arrhythmia, bradycardia, delirium.
Treatment:
- bradycardia: administration of atropine at a dose of 0.3–0.6 mg;
- arrhythmia: correction of gas levels in the blood;
- circulatory failure, arterial hypotension: reduction in the depth or cessation of general anesthesia, administration of plasma or plasma substitutes;
- hypertensive crisis: cessation of inhalation, increased oxygen supply, correction of metabolic acidosis and water-salt balance disorders, administration of antipyretics. If necessary, dantrolene is administered intravenously at a dose of 1 mg/kg (the total dose should not exceed 10 mg/kg) until the symptoms of the crisis stop. To avoid a relapse of the crisis, dantrolene is used for 1–3 days after surgery (orally or intravenously at a daily dose of 4–8 mg/kg in 4 divided doses);
- respiratory depression or inadequate postoperative ventilation: reducing the dose of anesthetic (if still used), maintaining the airway or artificial ventilation;
- delirium after recovery from general anesthesia: administration of a narcotic analgesic in small doses.
Using laughing gas for entertainment
Of all the varieties of dinitrogen oxide, it is food grade that is used for entertainment. N2O of this category does not contain additional impurities and additives, its use by humans is safe.
Food grade nitrous oxide comes in cylinders and is inhaled using balloons.
The company offers to buy food grade nitrous oxide at affordable prices. Our store operates 24/7, orders are delivered throughout Moscow around the clock, without restrictions. Selling food-grade nitrous oxide does not require a license; the product we offer is certified and safe for use.
What is laughing gas?
In the 18th century, American physicist Joseph Priestley discovered a new substance - nitrous oxide. As a result of the chemical reaction, a substance is heavier than air, which has a faint odor and a sweetish taste. During the experiments, Priestley, acting on copper with dilute nitric acid, for the first time obtained “nitrate air”
-
nitrogen oxide NO
(3Cu + 8HNO3 = 3Cu(NO3)2 +
2NO + 4H2O), and as a result of the reduction process of NO under the influence of moistened iron, the result was nitrous oxide N2O (6NO + 2Fe + 3H2O = 3N2O + 2Fe(OH)3). Nitrous oxide is used in medicine, the automotive and food industries. Later, the substance was called “laughing gas,” and its specific properties became the reason for the development of another area of substance abuse. Often, street “hucksters” pump nitrous oxide into balloons and sell this anesthetic drug under the guise of innocent colorful decorations for any holiday.
Indications for use
According to the instructions for use, nitrous oxide is used in the following cases:
- Inhalation combined anesthesia in combination with narcotic analgesics and muscle relaxants, carried out using special equipment;
- General anesthesia in operative gynecology, general surgery, dentistry and for pain relief during childbirth, which does not require muscle relaxation and deep anesthesia;
- Prevention of traumatic shock, as well as to enhance the analgesic and anesthetic effects of other drugs, including therapeutic analgesic anesthesia after surgery;
- Relief of pain in acute pancreatitis, myocardial infarction, acute coronary insufficiency;
- Switching off consciousness for pain relief during medical procedures.
Signs of use
The first signs may be abrasions on the body and bruises. If a person inhales a large amount of nitrous oxide, completely immersed in anesthesia, he simply will not be able to stand still, he will certainly fall to the ground and injure himself.
Read also: Why is sambuca set on fire?
In case of an overdose of laughing gas, asphyxia can occur - oxygen starvation and death can follow. The narcotic intoxication from laughing gas lasts approximately 30 minutes; if inhaled again, it can last up to several hours. It is similar in strength to alcohol, including:
- significant improvement in mood;
- slight dizziness and noise;
- a pleasant wave of warmth throughout the body;
- relaxation, laughter, fun;
- carelessness.
It is at these moments that a person experiences hallucinogenic manifestations, mostly pleasant ones. The colors around you become bright, the sounds become clear, the surge of energy goes off scale.
History of origin
Medical nitrous oxide is actively used in anesthesia. Its use has a beneficial effect on rapid recovery after surgery, while reducing various risks.
Food grade nitrous oxide can be used at home. Technical equipment is purchased exclusively for production purposes.
Nitrogen dioxide was first synthesized by Joseph Priestley back in 1772. He did this thanks to the effect of nitrogen acid on copper. Nitrous oxide was originally used as an analgesic in dentistry. However, after 40 years it began to be used in a huge list of procedures related to surgery. It began to be used not only for anesthesia, but also for pain relief.
Side effects
The use of Nitrous Oxide can cause side effects in the form of bradycardia, supraventricular arrhythmias, circulatory failure - during the period of introducing the patient into a state of general anesthesia.
Undesirable effects after recovery from anesthesia may include: drowsiness, nausea, vomiting, diffuse hypoxia, confusion, anxiety, agitation, nervousness, hallucinations, motor agitation. Long-term use (2 or more days) can lead to respiratory depression, impaired bone marrow function (leukopenia, pancytopenia), cause postoperative chills and hyperthermic crisis.