Causes of sigmoiditis
The anatomical shape of the intestine is often the cause of the development of sigmoiditis
Sigmoiditis as an independent disease develops due to the anatomical and physiological characteristics of the sigmoid colon. If we take into account that the sigmoid colon is the place where feces are finally formed, then it becomes clear why the disease occurs.
Feces can irritate this area of the intestine, which allows microdamage to develop and promotes inflammation.
Another cause of the disease is the curved shape characteristic of the sigmoid colon, which contributes to the long-term presence of intestinal contents in it and, as a result, irritation with feces for a longer period of time. This factor also significantly increases the risk of inflammation.
Also, the following factors are often attributed to the causes of inflammation:
- intestinal dysbiosis. Dysbacteriosis is a change in the composition of the intestinal microflora, resulting in the growth of aggressive bacteria that are not typical for this part of the body and a decrease in the protective function of the intestine.
- intestinal circulatory disorders or so-called ischemia. Most often, the cause of this is atherosclerosis, in which plaques appear in the vessels, clogging their lumens. Thus, the amount of blood passing through is reduced and the supply of nutrients to organs and tissues is disrupted. Places of necrosis (necrosis) appear in the intestines, which become foci of inflammation.
- intestinal infections. Due to the action of bacteria that provoke infections, toxic substances are produced that have a destructive effect on intestinal cells, causing ulcers or erosions to form. The sigmoid colon, due to its characteristics, most often undergoes this process.
- influence of ionizing radiation. Radiation contributes to the destruction of certain structures of the body's cells, which causes the formation of free radicals - toxic substances that have a negative effect on healthy cells.
- nonspecific intestinal ulcers, such as ulcerative colitis or Crohn's disease. The occurrence of this kind of disease is associated with the action of allergic factors and can cause intestinal damage akin to what happens with intestinal infections.
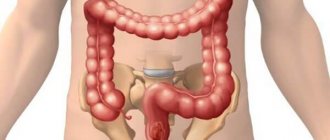
Description and functions of the sigmoid colon, possible causes of pain
Intestines: schematic representation
The sigmoid colon is part of the large intestine. At one end it connects to the lower colon, at the other - to the rectum. Most often, the sigmoid colon is located on the left side of the retroperitoneum. It is quite large and easily detected by palpation.
The sigmoid colon can be more than half a meter in length and about 4 cm in width. In women, the sigmoid colon is located directly behind the uterus, in men - behind the bladder. If for one reason or another the sigmoid colon becomes inflamed, the pain will intensify with palpation.
The main task of the sigmoid colon is the absorption of nutrients. This is where most of the vitamins and water entering the body are absorbed. The water is then distributed to other tissues and systems of the body. The formation of feces continues in the sigmoid colon, after which they enter the rectum.
Pain in the sigmoid colon is always an indicator of failure. The most common cause of pain is an inflammatory process, but other diseases and pathological processes are also possible. Only a doctor after an examination can accurately determine the cause of pain. The most common options:
- Sigmoiditis. This is inflammation of the sigmoid colon. Sigmoiditis is one of the types of colitis. This disease is always accompanied by abdominal pain of varying intensity. In the acute form of the disease, the pain can be severe, sharp and accompanied by diarrhea.
- Diverticulosis of the sigmoid colon. Diverticulosis is a disorder of peristalsis of the sigmoid colon. If peristalsis weakens or, on the contrary, becomes too strong, stool does not pass further through the intestines, which causes many problems. All this is accompanied by pain, colic, and increased gas formation.
- Sigmoid colon cancer. Malignant tumors in the sigmoid colon lead to intestinal obstruction, severe pain, and nausea. Sigmoid colon cancer progresses slowly and is not characterized by the rapid appearance of metastases. Treatment is only surgical.
Types of sigmoiditis
Sigmoiditis can also become chronic
The course of sigmoiditis can be acute or chronic. Also, depending on the nature of the intestinal damage, the following types of this disease are distinguished:
- catarrhal. This is the mildest form of sigmoiditis, which is characterized by damage to only the upper layer of epithelial tissue.
- erosive. It occurs as a consequence of untreated catarrhal disease and has pronounced erosions - unprotected places on the intestinal mucosa, which appeared due to the destruction of the epithelium.
- ulcerative Prolonged irritation of erosions leads to the occurrence of deeper lesions of the mucous membrane - ulcers.
- perisigmoiditis. The most severe and dangerous form of sigmoiditis. The deeper layers of the intestinal walls become affected. The intestine loses its former mobility and an adhesive process occurs, as a result of which the intestinal loops are connected to each other.
Kinds
Like most inflammatory diseases, sigmoiditis can be acute or chronic. In addition, there are the following types, differing in the nature of intestinal damage:
- Catarrhal sigmoiditis. The mildest form, in which only the upper layer of the intestinal epithelium is damaged.
- Erosive sigmoiditis. It is a continuation of untreated catarrhal disease and is characterized by the destruction of the intestinal epithelium with the formation of erosions on it - open, unprotected areas of the mucous membrane.
- Ulcerative sigmoiditis. This form appears with prolonged irritation of erosions on the surface of the intestine, as a result of which they turn into ulcers - deeper defects of the mucous membrane.
- Perisigmoiditis. It is the most severe form of the disease. Through the ulcerative surface, inflammation penetrates into the deep parts of the intestinal wall, reduces its mobility, and the adhesive process begins in the abdominal cavity (the process of connecting intestinal loops to each other).
Symptoms of sigmoiditis
Due to the variety of forms and causes of sigmoiditis, symptoms can vary greatly, but there are three main manifestations characteristic of any type of disease:
Pain in the left iliac region (lower left corner of the abdomen). The pain is intense and can often radiate to the leg or lower back. It should be remembered that the sigmoid colon initially has high mobility, which may result in a change in the localization of pain closer to the central line of the abdomen, or higher, towards the diaphragm.
Changes in the frequency and nature of stool. Diarrhea occurs most often, constipation occurs somewhat less frequently. Sigmoiditis is characterized by an increase in the frequency of the urge to defecate, which is explained by intestinal irritation. Feces are most often liquid, have a sharp, unpleasant odor, and you can see blood, mucus or pus in them.
Deterioration of the patient's general condition. Since during a long course of the disease the human body is depleted, loss of body weight, decreased performance and general well-being, and various sleep disorders are possible.
Characteristic symptoms of sigmoiditis
Symptoms of sigmoiditis can be varied and vary greatly with different types of this disease. But still, there are three main manifestations of it:
- pain in the left iliac region. The pain intensity is high, often radiating to the lower back or leg. It is important to remember that the sigmoid colon is very mobile. Therefore, pain can be localized not only in the lower left corner of the abdomen, but also move closer to its center or even the diaphragm.
- the occurrence of stool disorders. Diarrhea occurs most often, constipation occurs less frequently. The urge to defecate becomes more frequent, which is simply explained by intestinal irritation and is also characteristic of this disease. In this case, the feces have a liquid consistency, often with admixtures of blood, mucus, pus, and an unpleasant, pungent odor.
- deterioration of the patient's general well-being. Due to the depletion of the body by a long-term disease, weight loss, sleep disturbances, deterioration and decreased performance may occur.
On methods of diagnosis and conservative treatment of anorectal diseases
Despite the fact that anorectal pathology has long attracted the attention of researchers, until now it has received insufficient attention. Basically, surgical methods of treatment are described, to the development of which A. V. Vishnevsky, A. N. Ryzhikh, M. Kh. Levitan, Zh. M. Yukhvidova, V. D. Fedorov, G. I. Vorobyov made a significant contribution.
The purpose of our article is to present traditional and modern methods of diagnosis and conservative treatment of anorectal diseases.
Diagnostics
Since ancient times, methods such as examination of the anus and digital examination of the rectum have been used to diagnose anorectal pathology. Later, rectoscopy began to be used. These techniques have not lost their importance to this day.
Examination of the anus is the first stage of examination if anorectal pathology is suspected. The method makes it possible to identify external hemorrhoids and hemorrhoidal fimbriae, external openings of fistulas, and anal fissures. With idiopathic anal itching and proctitis, weeping and excoriation of the skin around the anus can be observed; with paraproctitis localized in the subcutaneous tissue, hyperemia and swelling of the perianal skin can be observed. When examining the anus, you can see an anal polyp, prolapse of internal hemorrhoids, the mucous membrane of the anal canal, or all layers of the rectum.
Digital examination has always been and remains a mandatory part of a proctological examination. The importance of this method was repeatedly emphasized in his speeches by Academician V. Kh. Vasilenko. He was supported in this by Professor V.M. Mysh, who wrote: “The scope of diagnostic capabilities of sigmoidoscopy is limited to diseases of the intestine itself, while digital examination through the rectum is an extremely valuable and widely available method for studying both the intestine itself and a number of adjacent organs.” In the diagnosis of rectal cancer, digital examination is of particular importance and makes it possible in 88% of cases to detect a tumor, determine the degree of its displacement, the distance from the anus (which is important for choosing the method of surgical intervention), and the narrowing of the intestinal lumen. In addition to examining the rectum, this method determines the condition of neighboring organs (prostate gland in men, cervix and posterior surface of the uterine body in women).
Anoscopy - examination of the anal canal and lower ampullary part of the rectum using rectal mirrors is also included in the group of methods for mandatory examination of patients with anorectal pathology. During this examination, hemorrhoids can be identified, true polyps can be differentiated from hypertrophied anal papillae, which are hyperplasia of the mucous membrane in the area of Morganian crypts, resulting from chronic inflammation in anal fissures, hemorrhoids or proctitis.
Sigmoidoscopy has long been considered the main method for visual examination of the rectum and distal sigmoid. In subsequent years, the rigid sigmoidoscope was replaced by flexible fiberglass sigmoidoscopes, which make it possible to examine the entire left part of the colon, the most common location of tumors.
Total colonoscopy is performed in cases where sigmoidoscopy reveals polyps or malignant tumors in the rectum or sigmoid colon, when an inflammatory process is detected that is not limited to the distal colon, or there is a pathological discharge (mucus, blood, pus) in the intestinal lumen coming from proximal sections.
Ultrasonography. Currently, to recognize complicated rectal pathology, ultrasound devices equipped with rectal sensors have begun to be used, which makes it possible to obtain information about the transmural spread of the inflammatory process in the distal colon, the presence of infiltrates, fistulas and abscesses.
Thus, today colonoscopy and ultrasound using rectal sensors have joined the arsenal of the above-described and well-known diagnostic methods.
Characteristics of anorectal diseases
Hemorrhoids are one of the most common diseases; it affects 10% of the population. Among proctological diseases, hemorrhoids account for 40%.
The views of researchers on the etiology of hemorrhoids are contradictory. Congenital insufficiency of the venous system, venous stagnation, constipation, and disruption of the rectal sphincter mechanism were cited as causative factors. At the same time, none of the hypotheses based on the pathology of the venous system for a long time could explain the origin of the main symptom of hemorrhoids - the release of scarlet blood. In 1975, W. Thomson experimentally proved the existence of arterial and venous components of the hemorrhoid [9]. He formulated the cause of hemorrhoids as a primary weakness of the epithelium of the anal canal, leading to slipping and displacement of the anal “cushions,” which can occur with chronic constipation or prolonged straining during bowel movements [10].
Summarizing the research conducted to study the pathogenesis of hemorrhoids, G. I. Vorobyov and co-authors came to the conclusion that “the leading factors in the pathogenesis of hemorrhoids are dysfunction of the vessels that provide blood flow through the cochlear arteries and outflow through the efferent veins” [1].
There are several classifications of hemorrhoids [1, 4–6].
According to one of the latest classifications, hemorrhoids are divided according to their clinical course into acute and chronic, and according to their form into internal, external and combined. The chronic course of hemorrhoids, in turn, is divided into four stages: Stage I - discharge of blood from the anus without prolapse of hemorrhoids; II - loss of nodes with spontaneous reduction into the anal canal (with or without bleeding); III - periodic prolapse of nodes with the need for their manual reduction into the anal canal, stage IV - constant prolapse of hemorrhoids along with the rectal mucosa [1].
Harbingers of hemorrhoids over a long period of time (from several months to several years) may be discomfort in the anus or anal itching. The first and main symptom of the disease is anorectal bleeding of varying severity - from scanty bloody traces on toilet paper or stool to massive bleeding, leading to anemia in 1% of cases. The blood is usually bright red, but may be dark if it accumulates in the rectal ampulla. At the very beginning of the act of defecation, blood accumulated in the rectum can be released in the form of clots. More often, patients note the release of blood in the form of drops or a splashing stream. Occasionally, bleeding is observed outside the act of defecation. Hemorrhoids are also characterized by pain in the anus, which occurs during bowel movements, walking, and poor diet (eating spicy foods, alcoholic drinks). The cause of pain may be changes in the perianal area with external hemorrhoids or associated complications (anal fissure, thrombosis of the external hemorrhoidal plexuses). Anal itching occurs quite often with hemorrhoids and is a consequence of excessive mucus secretion, contamination of the anal area with blood and fecal particles. This constantly causes a feeling of wetness around the anus and soiling of the underwear. As a result, scratching appears and excoriation of the skin of the perianal area occurs (Fig. 1).
At the initial stages of the disease, conservative therapy is carried out. Attention is paid to nutrition. Alcoholic drinks and irritating foods contribute to increased hemorrhoidal bleeding, so alcohol, seasonings, spicy and salty foods are excluded from food. After defecation and toileting the anus, soft suppositories are inserted into the anus. For a long period of time, in proctological practice, suppositories of the following composition have been deservedly used for these purposes: Extr. Belladonnae 0.015; Novocaini 012; Xeroformi 0.1; But. Cacao 1.7. In case of bleeding, add S. Adrenalini 1:1000 gtt to the above composition. IY. Other candles listed below are currently in use.
In acute hemorrhoids and hemorrhoids complicated by thrombosis of the hemorrhoid, conservative therapy is first carried out, aimed at eliminating the inflammatory process and regulating stool. On the first day, cold is prescribed to the perineal area, in subsequent days - warm sitz baths with a weak solution of manganese after stool and rectal suppositories of the above composition, as well as suppositories or ointment Ultraproct, Posterizan, Proctoglivenol, Relief, Relief Ultra or Advance. These drugs are prescribed rectally in the form of cream or suppositories 2 times a day after stool and at night. In addition to local therapy, drugs that have a venotonic and angioprotective effect are prescribed orally. One of these drugs, Detralex, belongs to the group of flavonoids and is a combination of diosmin and hesperidin. The drug helps reduce the distensibility of veins, increase their tone, reduce stagnation and improve microcirculation. Detralex is prescribed 6 tablets per day for the first 4 days, then 4 tablets per day for the next 3 days.
The second drug in the same direction, Phlebodia 600, is non-micronized diosmin. The drug is prescribed according to the following regimen: 2–3 tablets per day with meals for 7 days, then, if necessary, treatment can be continued at 1 tablet 1 time per day for 1–2 months. For the same purpose, Diovenor 600, an analogue of Phlebodia 600, is used.
In case of prolapse of nodes, frequent exacerbations that do not respond to conservative therapy, and heavy recurrent bleeding, surgical treatment is indicated (Fig. 2). According to the Mayo Clinic, the most satisfactory results were obtained by alloying the nodes with a latex washer and hemorrhoidectomy.
Anal fissure ranks second among anorectal diseases and is a linear or triangular mucosal defect 1.0–1.5 cm long, located near the transitional fold, above the Hilton line. The origin of the fissure is associated with many reasons, but the most important factor is trauma to the mucous membrane of the anal canal by feces, foreign bodies, and damage to it during childbirth. A predisposing factor may be hemorrhoids.
A sharp crack has a slit-like shape, smooth, even edges. Its bottom is made up of sphincter muscle tissue. With a long course of the pathological process, connective tissue grows along the edges of the crack. Its bottom is covered with granulations. In the area of the outer edge of the crack, excess tissue forms the anal (sentinel) tubercle. Thus, an acute fissure turns into a chronic one, which is an ulcer with scarred edges and a scarred bottom (Fig. 3).
Sometimes an acute crack heals on its own, but more often it becomes chronic. As a rule, there is only one crack and most often it is located on the back wall of the anal canal. Occasionally you can observe two cracks that are located one above the other. It is necessary to distinguish true cracks from various superficial damage to the perianal skin. The most common localization of cracks (at 12 o'clock on the posterior wall and at 6 o'clock on the anterior wall) is explained by the structural features of the anal sphincter. It is at 6 and 12 o'clock that the blood supply conditions are worse, and there is a great danger of injury to the mucous membrane during the passage of feces during defecation due to pressure on the posterior and anterior commissures. Trauma to the mucous membrane leads to acute pain due to irritation of numerous nerve endings and sphincter spasm. A vicious circle is formed - an anal fissure leads to a sharp pain syndrome, pain syndrome leads to sphincter spasm, sphincter spasm prevents the healing of the fissure.
A triad of symptoms characteristic of the clinical picture of an anal fissure is pain during and especially after defecation, sphincter spasm and scanty blood discharge. There are differences in the clinical picture of acute and chronic fissures. With an acute fissure, the clinical manifestations are pronounced. The main symptom is acute pain after defecation, lasting several hours. Sphincter spasm increases pain. The pain becomes throbbing. Blood is detected as droplets on the surface of the stool. Due to sphincter spasm and a feeling of fear before defecation, stool is delayed. Taking laxatives increases pain.
With a chronic fissure, the pain is less acute, its duration after stool is 5–10 minutes. Sphincter spasm is mild. When a chronic anal fissure is complicated by marginal fistulas, purulent discharge, itching, and irritation of the perianal skin appear. A chronic crack is characterized by a cyclic course. It can heal, but at the slightest strain or physical stress it opens again.
Detecting an anal fissure is not difficult. Anamnesis and characteristic complaints suggest its existence. The diagnosis becomes clear already upon examination of the anus. When spreading the buttocks, a crack or guard tubercle is discovered at its outer edge. If the crack is not visible upon examination, then a digital examination of the rectum should be carefully performed. In this case, a compaction will be detected on the affected wall of the anal canal. Sigmoidoscopy in the acute period should not be performed due to severe pain and sphincter spasm. In cases where it is necessary to differentiate an anal fissure from other diseases, the study can be carried out after a preliminary injection of 4-5 ml of 1% novocaine solution under the fissure. Differential diagnosis is carried out with coccydynia and the anal form of Crohn's disease.
Conservative treatment of an acute fissure involves, first of all, eliminating pain, sphincter spasm and normalizing stool. To normalize stool, Mucofalk can be used, containing hydrophilic fibers from the outer shell of plantain seeds that retain water. As a result, the volume of stool increases and its consistency becomes softer. The drug is well tolerated by patients and does not increase pain in the abdomen and anus, which is characteristic of many laxatives. A diet limiting spicy and irritating foods is recommended. Once a day, cleansing enemas of boiled water at room temperature are given. In this case, the enema tip is thickly lubricated with petroleum jelly and inserted along the edge of the anus opposite to the one along which the crack is located.
After cleansing the intestines, take warm (38 °C) sitz baths with a weak solution of manganese for 10 minutes. Self-stool should be avoided during this period due to the risk of increased pain and spasm. Laxatives, as mentioned above, are contraindicated, as they can increase pain. Then soft-based suppositories (Relief, Relief Advance, Ultraproct, Posterisan) are inserted into the rectum. Treatment with suppositories is carried out until the crack is completely epithelized. Cleansing enemas, as a rule, are given during the first 10 days from the onset of the disease, after which the stool is independent.
When conservative therapy is ineffective, surgical treatment is resorted to, which consists of excision of the fissure and sphincterotomy. In recent years, closed lateral subcutaneous sphincterotomy has been proposed for the treatment of uncomplicated anal fissure [3, 7], after which pain disappears faster and there are fewer relapses.
Paraproctitis is inflammation of the tissue surrounding the rectum. Among proctological diseases, paraproctitis accounts for 15%. There are three fascial-cellular spaces: subcutaneous, ischiorectal and pelvic rectal. Accordingly, paraproctitis is divided into subcutaneous, submucosal, ischiorectal and pelvic-rectal. The disease is caused by various microorganisms that penetrate into the cellular spaces from the rectum through the anal glands, damaged mucous membrane, as well as hematogenously or lymphogenously from neighboring organs affected by the inflammatory process.
Of particular importance in the pathogenesis of paraproctitis is direct damage to the mucous membrane of the rectum in the area of the posterior wall of the anal canal, where wide and deep crypts are located, which are the entrance gates of infection. From 6 to 8 ducts of the anal glands open into each crypt. Through them, the infection spreads to the perirectal tissue spaces. According to the clinical course, paraproctitis is divided into acute and chronic. Acute paraproctitis is a purulent inflammation (abscess) of the peri-rectal tissue. Chronic paraproctitis is a consequence of acute inflammation. This is a pararectal fistula, formed after spontaneous opening of an abscess or opening it surgically. The internal opening of a pararectal fistula is a defect in the rectum. The external opening is located on the skin of the perineum (Fig. 4).
In some cases, several fistula tracts and several external openings of the fistula can be observed. The disease begins with a prodromal period (up to three days), during which weakness, headache, and general malaise may be observed. Then chills, fever, and pain in the perineal area appear. When the cellular spaces of the small pelvis are affected by the type of phlegmon, the clinical picture is dominated by general symptoms caused by intoxication. As the process is limited and an abscess is formed, the intensity of the pain increases. It takes on a pulsating character.
The greatest importance in the diagnosis of acute paraproctitis is examination of the anal area and digital examination of the rectum. Upon examination, attention is drawn to hyperemia of the skin in the perineal area on the affected side. Palpation of the perineum is painful, fluctuation can be detected. Digital examination is also painful in many cases and should be performed with caution. This technique should not be neglected, since the information obtained through digital examination can be very valuable for recognizing almost all forms of acute paraproctitis.
Treatment of acute paraproctitis, carried out immediately after diagnosis, is surgical. Basic principles of treatment: 1) opening the abscess; 2) elimination of the internal opening through which the abscess cavity communicates with the rectum.
Chronic paraproctitis, as mentioned above, is a consequence of acute paraproctitis. If, when opening the abscess, the internal opening in the rectum is not eliminated, a rectal fistula may subsequently form. A rectal fistula is characterized by the presence of one or more external openings in the skin of the perineum. Pus, feces and gases may be released from the external openings of the fistula tract. The general condition of patients with chronic paraproctitis suffers little. Pain appears only when the process worsens. Depending on the location of the fistula tract in relation to the sphincter muscle, four types of fistulas are distinguished: subcutaneous-submucosal (intrasphincteric), transsphincteric, complex (extrasphincteric) and incomplete. The latter have only an internal opening in the rectum and do not have an external opening in the perineum. The source of their formation is often an anal fissure.
In the diagnosis of chronic paraproctitis, the same methods are used as for acute paraproctitis. Digital examination of the rectum makes it possible to judge the tone of the sphincter and, in some cases, detect the internal opening of the fistula. Be sure to perform probing with a button probe, which is inserted into the fistula tract through its external opening (Fig. 5).
Using a probe, the direction of the fistula tract and its relationship to the sphincter muscle are determined. Fistulography is a mandatory x-ray examination of rectal fistulas. A test with a dye is used to determine the patency of the fistula tract, the location of the internal opening and purulent cavities in the tissue. Sigmoidoscopy is performed to detect concomitant diseases and high-lying internal fistula openings.
Treatment of all types of fistulas is surgical. The operation can be considered radical only if the opening in the rectum is eliminated by excision of the fistula tract.
Proctitis is an acute or chronic inflammation of the mucous membrane of the rectum or rectum and sigmoid colon (proctosigmoiditis). Acute proctitis most often has a specific cause. It can be caused by trauma, foreign bodies, mechanical irritation during prostate massage, chemical and thermal burns, and frequent cleansing enemas. The disease may be a consequence of radiation therapy for malignant tumors of the pelvic organs (radiation proctitis) or infections (gonorrheal, dysenteric proctitis). The development of proctitis can be facilitated by diseases of neighboring organs (hemorrhoids, anal fissure, paraproctitis, Douglas pouch abscess, prostatitis, cystitis, vulvovaginitis, etc.). Long-term proctitis and proctosigmoiditis that cannot be treated conservatively should, first of all, be differentiated from distal forms of ulcerative colitis [2].
Acute proctitis has a sudden onset, may be accompanied by fever, chills, is characterized by inflammatory changes in the rectal mucosa, clinically manifested by a frequent urge to defecate, tenesmus due to constipation, a burning sensation and a sensation of a “foreign body” in the anus. Examination of the rectum with a finger and sigmoidoscopy are poorly tolerated by patients due to severe pain. The rectal mucosa is sharply hyperemic, swollen, and bulges into the lumen. The vascular pattern is blurred or absent.
Chronic proctitis, as a rule, is a consequence of an untreated acute disease or has a specific nature. Clinically, the disease is manifested by a periodic feeling of discomfort in the rectum, a feeling of incomplete emptying, periodic exacerbations, accompanied by frequent stools mixed with mucus (sometimes blood), and tenesmus. Endoscopy reveals hyperemia of varying severity and swelling. The diagnosis requires the exclusion of infectious, parasitic and other diseases.
Treatment of acute proctitis is conservative. First of all, you need a diet that excludes all irritating foods and alcohol. After complete cleansing of the intestines, 100.0 ml of warm chamomile infusion (temperature 37–38 °C) is injected into the intestine for therapeutic purposes. An oil enema is given at night (50–75 ml of warm -37–38 °C vegetable oil). Starting from the second week of the disease, morning medicinal chamomile enemas are replaced with enemas of 0.3–0.5% collargol solution. The concentration of the solution is determined by the intensity of the inflammatory process in the intestine. Oil evening microenemas are continued for 14 days. The general course of treatment is 2–3 weeks. After a 10-day break, the course of treatment should be repeated to avoid relapse.
When treating chronic proctitis, the same remedies are recommended as for acute proctitis, but the course of conservative therapy is longer. The best effect is achieved by therapeutic microenemas from a collargol solution.
Anal itching is a pathological condition characterized by persistent scratching in the anus. It is necessary to clearly distinguish idiopathic (primary) anal itching and secondary, often accompanying diseases such as hemorrhoids, anal fissure, helminthic infestations, proctitis and proctosigmoiditis. While the causes of secondary anal itching are known, the etiology of primary (idiopathic) itching is not fully understood.
Often itching in the anus is the patient's main and only complaint. According to the clinical course, acute and chronic anal itching are distinguished. The acute form is characterized by a sudden onset, a constant course, significant intensity and local changes in the skin such as wet eczema with maceration, traces of scratching, hypertrophy of the perianal folds. With chronic itching, on the contrary, the onset is slow, the skin is often dry, thinned, sometimes depigmented, there are no traces of scratching at all or they look like thin linear abrasions.
Diagnosis of the causes of anal itching always begins with determining the type of itching (idiopathic or secondary). First of all, it should be determined whether itching is a symptom of the above diseases (secondary), whether its occurrence is associated with the intake of spicy or salty foods, alcohol, contact with harmful chemicals and radioactive substances, the presence of allergies, etc. From laboratory tests, a blood test is performed for sugar content and three-time stool analysis for worm eggs. In men, urethritis and prostatitis should be excluded; in women, vaginal itching should be excluded.
Treatment of secondary anal itching consists of treating the underlying disease. For idiopathic anal itching, treatment is purely symptomatic.
A diet excluding spicy foods and alcohol is recommended. Thorough toileting of the anus after each act of defecation. After this, ointments (Posterizan, Proctosedyl, Aurobin, Emalan) are injected into the rectum. At night, warm sitz baths with a solution of manganese and microenemas from a 0.33% solution of collargol -50.0 ml are prescribed. The course of treatment is 10 days. For the next 10 days, microenemas (50 ml) of warm vegetable oil are prescribed.
Methods of conservative treatment of anal pathology are being improved as new drugs are developed. From this point of view, one should pay attention to medical collagens, which have become used in the complex therapy of many diseases.
The problem of studying collagen as a new plastic material has long attracted the attention of researchers. At the origins of the development of this direction was Academician of the USSR Academy of Medical Sciences V.V. Kovanov. As a result, a new class of medicinal dosage forms was created - plastic materials with targeted action based on the natural biopolymer of collagen. Currently, medical collagen Emalan, which is a collagen hydrogel for problematic skin and wounds, has become widely used in practical healthcare. It successfully passed clinical trials at the Burn Center of the Research Institute of Emergency Medicine named after. N.V. Sklifosovsky (1995), at the Military Hospital named after. N. N. Burdenko (1999) and in the 72nd Central Clinic of the Ministry of Emergency Situations (2009). It has been established that Emalan reduces signs of inflammation. Under its influence, hyperemia, swelling, pain disappear, itching and peeling of the skin decreases or disappears. All of the above served as the basis for the use of this drug in the treatment of anal pathology.
Of the 112 we observed during 2009–2010. 54 patients had acute and chronic anal fissures, 50 had grade I and II hemorrhoids, and 8 had idiopathic anal itching.
When treating anal fissures, Emalan was included in complex therapy. It was introduced into the rectum on a suppository and in the form of applications to the anus for 3 weeks. In 70.3% of cases, positive dynamics were noted already on days 4–5 from the start of treatment. By the end of the third week, 31 patients had complete healing of the fissures. In patients suffering from hemorrhoids complicated by anal fissure and itching (50), by the end of the third week, against the background of complex therapy, a decrease in symptoms of the disease, such as itching, pain, burning, was observed. The drug turned out to be especially effective in the treatment of patients with idiopathic (8) and secondary (6) anal itching. While taking the drug (gel applications to the anal area 3-4 times a day), a decrease in scratching, maceration and skin hyperemia was noted. By the end of the two-week course, these changes completely disappeared.
Our first experience has shown that the domestic collagen gel Emalan can be successfully used in the complex treatment of acute and chronic anal fissures, proctitis, anal itching and complicated forms of hemorrhoids. The drug makes it possible to increase the effectiveness of therapy and helps reduce treatment time.
Literature
- Vorobyov G.I., Shelygin Yu.A., Blagodarny L.A. Hemorrhoids. M. // "Mitra-Press". 2002. 192 p.
- Grigorieva G. A. Anorectal pathology. Guide to gastroenterology // M.: Medicine. 1996. T3. 606–635 pp.
- Henry M. M., Swash M. Coloproctology and the pelvic floor // M.: Medicine. 1988 (translation from English). 451 p.
- Rivkin V.L., Kapuller L.L. Hemorrhoids // M.: Medicine. 1984. 751 p.
- Rivkin V.L., Dultsev Yu.V., Kapuller L.L. Hemorrhoids and other diseases of the anal canal // M.: Medicine. 1994. 239 p.
- Rivkin V. L., Kapuller L. L. Hemorrhoids. Constipation // M.: Medpraktika. 2000. 158 p.
- Fedorov V.D., Dultsev Yu.V. Proctology. M.: Medicine. 1984. 380 p.
- Haas PF, Fox TA, Haas GP The pathogenesis of haemorrhoids. Disease of the colon and rectum. 1984. T. 27. p. 442–450.
- Thomson WHF The nature of haemorrhoids // British J. of Surgery. 1975. T. 62. p. 542–552.
- Thomson WHF The nature and cause of haemorrhoids. Proceedings of the Royal Society of Medicine. 1975. T. 68. p. 574–575.
G. A. Grigorieva , Doctor of Medical Sciences, Professor S. V. Golysheva First Moscow State Medical University named after. I. M. Sechenova , Moscow
Contact information for authors for correspondence
Diagnosis of sigmoiditis
Diagnosis and treatment of sigmoiditis are the responsibility of doctors such as a gastroenterologist, therapist, surgeon, and infectious disease specialist. Diagnosis is possible after differential diagnosis to exclude other inflammatory processes in the intestines, such as paraproctitis, ulcerative colitis, as well as infectious diseases - dysbacteriosis, dysentery, cholera.
Diagnostics includes the following studies:
Prognosis and complications
With proper treatment of sigmoiditis, in most cases it is possible to achieve complete recovery, but it should be understood that the treatment process is long and is accompanied by a lot of dietary restrictions.
If left untreated, inflammation may spread to adjacent sections of the intestine, most often to the rectum (proctitis).
Also, as inflammation progresses, the tightness of the intestine may become compromised, resulting in peritonitis - inflammation of the abdominal cavity, requiring extensive surgical intervention.
How to treat sigmoiditis
Painful sensations as a symptom of sigmoiditis
Treatment of sigmoiditis is carried out based on the causes of its occurrence. In case of sigmoiditis as a result of intestinal infections, treatment is based on antibacterial therapy with drugs such as Biseptol, Cefran, Tetracycline, Ampicillin.
It is mandatory to prevent dysbiosis with Bifidobac, Lactobacterin, etc. If the disease is chronic, the patient is also prescribed intestinal antiseptics, such as Smecta or Intetrix.
Sigmoiditis, provoked by nonspecific inflammatory bowel diseases, is treated with anti-inflammatory drugs that have an effect on the underlying disease: Sulfasalazine, Salazoperidazine, Prednisolone.
General intoxication processes are eliminated by infusion therapy. For this purpose, solutions of glucose, blood plasma and, if necessary, treatment of anemia, iron preparations are used. In order to restore normal microflora, bacterial medications and preparations are also prescribed.
Treatment of ischemic sigmoiditis has the same features as the treatment of sigmoiditis caused by nonspecific diseases. If therapy does not bring the desired effect, surgery may be prescribed to plasticize the vessels that feed the intestines.
In addition, the patient is prescribed a special diet 4, the peculiarity of which is the exclusion of smoked, fried, spicy foods, alcohol from the diet and minimizing the consumption of fats, salt and carbohydrates. Also a prerequisite for the diet is chopping food before eating it.
Most often, treatment of sigmoiditis is long-term and can last from 1 to 3 months with 1-2 courses of medical therapy.
Planned surgical treatment of diverticulosis
As a rule, after a single attack of diverticulitis, surgical treatment is not indicated, but may be recommended for persons under 45 years of age. The decision to perform an operation is made individually.
After successful conservative treatment of two or more episodes of diverticulitis, elective surgery is recommended. The indications for elective surgery are limited by the high anesthetic and surgical risks identified during examination of the patient.
Elective surgical treatment is mandatory after conservative treatment of complicated diverticulitis.
Planned surgical treatment is indicated in the presence of fistulas.
FEATURES OF SIGMOIDITIS
The main symptoms of the disease depend on the type of pathology, the severity of the inflammatory process and the presence of complications. Among the most characteristic manifestations of the disease are:
- lower abdominal pain;
- violation of the act of defecation (diarrhea);
- general weakness and malaise.
With sigmoiditis, pain radiates to the left leg or lumbar region. In such patients, blood inclusions are often detected in the stool, and the stool itself is foul-smelling and has a foamy consistency.
TREATMENT OF SIGMOIDITIS
Treatment of inflammation in the sigmoid colon is a rather lengthy and expensive process. The results of therapy largely depend on how diligently the patient follows the recommendations of the attending physician.
The main thing is to stop the inflammation and restore the mucous membrane of the gastrointestinal tract, so the emphasis should be on antibacterial drugs and gastroenteroprotectors (rebamipide, rebagit). Analgesics and antispasmodics will help cope with pain, probiotics will help restore the microflora.
The basis of treatment for sigmoiditis is:
- drugs to protect and restore the intestinal mucosa (gastroenteroprotectors);
- adsorbents for removing waste;
- antibacterial drugs to eliminate the source of infection in the sigmoid colon;
- drugs to reduce stomach acidity;
- probiotics to normalize normal intestinal microflora;
- analgesics to relieve pain;
- antispasmodics to reduce spastic phenomena in the large intestine;
- in severe forms of the disease, patients are prescribed intravenous injections of detoxification solutions and a mixture of amino acids.
Diet plays an important role in the treatment of sigmoiditis. Limiting the consumption of fatty foods and carbohydrates eliminates the processes of fermentation and putrefaction that cause irritation of the intestinal walls. Patients suffering from sigmoiditis are advised to eat frequent and small portions of liquid, viscous meals without lumps or other solid particles. For sigmoiditis accompanied by severe diarrhea, diet No. 4 is indicated. Due to insufficient calories, this diet is usually prescribed for no more than 7 days. In severe cases, patients with sigmoiditis are advised to fast and drink plenty of fluids for 1-2 days.