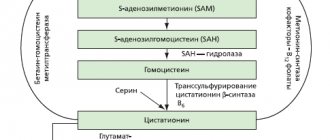
Compound
| Capsules | 1 caps. |
| active substances: | |
| amlodipine besylate | 6.95/6.95/13.9/13.9 mg |
| (corresponds to amlodipine - 5/5/10/10 mg) | |
| ramipril | 5/10/5/10 mg |
| excipients: crospovidone - 20/40/40/40 mg; hypromellose - 1.18/2.36/2.36/2.36 mg; MCC - 114.82/229.64/229.64/229.64 mg; glyceryl dibehenate - 2.05/4.1/4.1/4.1 mg | |
| Capsules, 5 mg+5 mg; hard gelatin capsule (CONI-SNAP 3), cap and base color code - 51072: brilliant blue dye (E133), charming red dye (E129), titanium dioxide, gelatin | |
| Capsules, 5 mg+10 mg; hard gelatin capsule (CONI-SNAP 0), cap and base color code - 51072/37350: cap - titanium dioxide; brilliant blue dye (E133), charming red dye (E129); gelatin; base - titanium dioxide; red iron oxide dye (E172); gelatin | |
| Capsules, 10 mg+5 mg; hard gelatin capsule (CONI-SNAP 0), cap and base color code - 33007/37350: cap - titanium dioxide; Azorubine dye (E122); indigo carmine (E132); gelatin; base - titanium dioxide; red iron oxide dye (E172); gelatin | |
| Capsules, 10 mg+10 mg; hard gelatin capsule (CONI-SNAP 0), cap and base color code - 33007: azorubine dye (E122), indigo carmine (E132), titanium dioxide, gelatin |
Analogs
Level 4 ATC code matches:
Actaparoxetine
Plizil
Fluxen
Paroxin
Surlift
Asentra
Elycea
Fluoxetine
Lenuksin
Escitalopram
Selectra
Stimuloton
Citalopram
Cipramil
Zoloft
Paroxetine
Prozac
Paxil
Rexetine
Fevarin
Paxil , Apo-paroxetine , Paroxetine , Plizil , Actaparoxetine , Rexetine , Sirestill .
Description of the dosage form
Capsules 5 mg + 5 mg: hard gelatin CONI-SNAP 3, self-closing, with a light burgundy base and cap.
Capsules 5 mg + 10 mg: hard gelatin CONI-SNAP 0, self-closing, with a light pink base and a light burgundy cap.
Capsules 10 mg + 5 mg: hard gelatin CONI-SNAP 0, self-closing, with a light pink base and a dark burgundy cap.
Capsules 10 mg+10 mg: CONI-SNAP 0 hard gelatin capsules, self-closing, with a dark burgundy base and cap.
Capsule contents: a mixture of granules and powders of white or almost white color, odorless or almost odorless.
Pharmacodynamics
Amlodipine
Dihydropyridine derivative. By binding to dihydropyridine receptors, it blocks slow calcium channels, inhibits the transmembrane transition of calcium into vascular and cardiac smooth muscle cells (to a greater extent into vascular smooth muscle cells than into cardiomyocytes). It has antihypertensive and antianginal effects.
The mechanism of the antihypertensive effect of amlodipine is due to a direct relaxing effect on vascular smooth muscle.
Amlodipine reduces myocardial ischemia in the following two ways:
1. Dilates peripheral arterioles and thus reduces TPSS (afterload), while heart rate remains virtually unchanged, which leads to a decrease in energy consumption and myocardial oxygen demand.
2. Dilates coronary and peripheral arteries and arterioles in both normal and ischemic areas of the myocardium, which increases the supply of oxygen to the myocardium in patients with vasospastic angina (Prinzmetal angina) and prevents the development of coronary spasm caused by smoking.
In patients with arterial hypertension (AH), a daily dose of amlodipine provides a decrease in blood pressure over 24 hours (both in the supine and standing positions). Due to its slow onset of action, amlodipine does not cause a sharp decrease in blood pressure.
In patients with angina pectoris, a single daily dose of the drug increases the duration of physical activity, delays the development of the next attack of angina and ST segment depression (by 1 mm) during physical activity, reduces the frequency of angina attacks and the need for nitroglycerin.
The use of amlodipine in patients with coronary artery disease. In patients with cardiovascular diseases (including coronary atherosclerosis with damage from one vessel to stenosis of 3 or more arteries and atherosclerosis of the carotid arteries), who have had myocardial infarction (MI), percutaneous transluminal angioplasty of the coronary arteries (PCA) or suffering from angina pectoris), the use of amlodipine is prevented the development of thickening of the intima-media of the carotid arteries, significantly reduces mortality from cardiovascular causes, MI, stroke, TLP, coronary artery bypass grafting, leads to a decrease in the number of hospitalizations for unstable angina and progression of CHF, reduces the frequency of interventions aimed at restoring coronary blood flow .
Use of amlodipine in patients with heart failure (HF). Amlodipine does not increase the risk of death or the development of complications and deaths in patients with CHF III–IV functional class (FC) according to the New York Heart Association (NYHA) classification during therapy with digoxin, diuretics and ACE inhibitors. In patients with NYHA class III–IV CHF of non-ischemic etiology, when using amlodipine, there is a risk of pulmonary edema. Amlodipine does not cause adverse metabolic effects, incl. it does not affect the lipid profile.
Ramipril
Ramiprilat, formed with the participation of liver enzymes, the active metabolite of ramipril, is a long-acting inhibitor of the enzyme dipeptidyl carboxypeptidase I (synonyms - ACE, kininase II). In the blood plasma and tissues, this enzyme kininase II catalyzes the conversion of angiotensin I into the active vasoconstrictor substance angiotensin II, and also promotes the breakdown of bradykinin. Reducing the formation of angiotensin II and inhibiting the breakdown of bradykinin leads to vasodilation and a decrease in blood pressure. An increase in the activity of the kallikrein-kinin system in the blood and tissues determines the cardioprotective and endothelioprotective effects of ramipril due to the activation of the PG system and, accordingly, an increase in the synthesis of PGs that stimulate the formation of nitric oxide (NO) in endothelial cells. Angiotensin II stimulates the production of aldosterone, so taking ramipril leads to a decrease in aldosterone secretion and an increase in the content of potassium ions in the serum.
By reducing the content of angiotensin II in the blood, its inhibitory effect on renin secretion by type of negative feedback is eliminated, which leads to an increase in plasma renin activity.
It is assumed that the development of some undesirable reactions (in particular dry cough) is associated with an increase in bradykinin activity.
In patients with hypertension, taking ramipril leads to a decrease in blood pressure in the supine and standing positions, without a compensatory increase in heart rate. Ramipril significantly reduces peripheral vascular resistance, causing virtually no changes in renal blood flow and glomerular filtration rate. The antihypertensive effect begins to appear 1–2 hours after oral administration of a single dose of the drug, reaching its greatest value after 3–6 hours, and persists for 24 hours. With a course of administration, the antihypertensive effect can gradually increase, usually stabilizing by the 3–4th week taking the drug regularly and then maintaining it for a long time. Sudden discontinuation of the drug does not lead to a rapid and significant increase in blood pressure (no withdrawal syndrome).
In patients with hypertension, ramipril slows down the development and progression of myocardial and vascular wall hypertrophy.
In patients with CHF, ramipril reduces the peripheral vascular resistance (reducing afterload on the heart), increases the capacity of the venous bed and reduces the filling pressure of the left ventricle (LV), which accordingly leads to a decrease in preload on the heart. In these patients, when taking ramipril, there is an increase in cardiac output, ejection fraction and improved exercise tolerance.
In diabetic and non-diabetic nephropathy, taking ramipril slows the rate of progression of renal failure and the onset of end-stage renal failure and thereby reduces the need for hemodialysis or kidney transplantation. In the initial stages of diabetic or non-diabetic nephropathy, ramipril reduces the severity of albuminuria.
In patients with a high risk of developing cardiovascular diseases due to the presence of vascular lesions (diagnosed coronary artery disease, a history of occlusive peripheral artery disease, a history of stroke) or diabetes mellitus with at least one additional risk factor (microalbuminuria, hypertension, increased total cholesterol, decreased HDL cholesterol, smoking) the addition of ramipril to standard therapy significantly reduces the incidence of myocardial infarction, stroke and mortality from cardiovascular causes.
In addition, ramipril reduces overall mortality rates, as well as the need for revascularization procedures, and slows down the onset or progression of CHF.
In patients with HF that developed in the first days of acute myocardial infarction (AMI) (days 2–9), taking ramipril, starting from days 3 to 10 of AMI, reduces the risk of mortality (by 27%), the risk sudden death (by 30%), the risk of progression of CHF to severe - NYHA class III–IV, resistant to therapy (by 27%), the likelihood of subsequent hospitalization due to the development of HF (by 26%). In the general population of patients, as well as in patients with diabetes mellitus, both with hypertension and with normal blood pressure, ramipril significantly reduces the risk of developing nephropathy and microalbuminuria.
Reviews of Adepress
The cause of depression , anxiety and panic attacks is insufficient activity of the GABAergic system or reduced GABA content. The drugs of choice in such cases are serotonin reuptake inhibitors, one of them is Paroxetine (Adepress).
Reviews from doctors boil down to the fact that Paroxetine is a new generation antidepressant, acts selectively and is not addictive, has wide indications for use, and combines a high therapeutic effect with safety of use.
Of the entire group of these drugs, Paroxetine is the strongest and most specific serotonin . It has a more balanced effect, and its advantage is the ability to be used on an outpatient basis in people with neurological and somatic pathologies . It is a dual-action drug used to treat anxiety and depression and treats a wider range of symptoms in these conditions. In addition, its action is manifested by a moderate stimulating effect.
Looking at reviews of Adepress on forums, we can conclude that the drug, with the correct dose, improves the condition of patients after the first week of treatment. It was effective in patients who had no effect from previous treatment. Taking tablets in the morning does not affect the duration of sleep. Everyone notes the ease of use (once a day), which is important for patients receiving maintenance outpatient treatment. Patients note good tolerability even with long-term maintenance therapy. Undesirable side reactions are rare and mild.
There were isolated cases of headache, dry mouth and decreased blood pressure when changing the position of the body.
Pharmacokinetics
Amlodipine
After oral administration in therapeutic doses, amlodipine is well absorbed, the time to reach Cmax in blood plasma after oral administration is 6–12 hours. Absolute bioavailability is 64–80%. Vd is approximately 21 l/kg. The binding to plasma proteins is approximately 97.5%. Food intake does not affect the absorption of amlodipine. The drug penetrates the BBB.
T1/2 from blood plasma is about 35–50 hours, which corresponds to the administration of the drug once a day. In patients with liver failure and severe CHF, T1/2 increases to 56–60 hours.
Total clearance - 0.43 l/h/kg.
Stable Css (5–15 ng/ml) is achieved after 7–8 days of continuous administration of amlodipine; it is metabolized in the liver to form inactive metabolites. 10% of the parent drug and 60% of metabolites are excreted by the kidneys, and 20% through the intestines. Excretion into breast milk is unknown. Amlodipine is not removed during hemodialysis.
Special patient groups
Kidney failure. T1/2 from blood plasma in patients with renal failure increases to 60 hours. Changes in the concentration of amlodipine in blood plasma do not correlate with the degree of renal dysfunction.
Elderly patients. The time to reach Cmax and Cmax of amlodipine are practically no different from those in younger patients. In elderly patients suffering from CHF, there was a tendency to decrease the clearance of amlodipine, which leads to an increase in AUC and T1/2 up to 65 hours.
Ramipril
After oral administration, it is quickly absorbed from the gastrointestinal tract (50–60%). Eating slows down its absorption, but does not affect the degree of absorption. Ramipril undergoes extensive first-pass metabolism/activation (mainly in the liver, by hydrolysis), resulting in the formation of its only active metabolite, ramiprilat, whose ACE inhibitory activity is approximately 6 times greater than that of ramipril. In addition, as a result of the metabolism of ramipril, diketopiperazine, which does not have pharmacological activity, is formed, which is then conjugated with glucuronic acid. Ramiprilat is also glucuronidated and metabolized to diketopiperazic acid. The bioavailability of ramipril after oral administration ranges from 15% (for a dose of 2.5 mg) to 28% (for a dose of 5 mg). The bioavailability of ramiprilat after oral administration of 5 mg of ramipril is approximately 45% (compared to its bioavailability after intravenous administration in the same doses).
After oral administration of ramipril, the time to reach Cmax for ramipril and ramiprilat is 1 and 2–4 hours, respectively. The decrease in plasma concentrations of ramiprilat occurs in several stages: a distribution and elimination phase with a T1/2 of ramiprilat of approximately 3 hours, then an intermediate phase with a T1/2 of ramiprilat of approximately 15 hours, and a final phase with a very low concentration of ramiprilat in blood plasma and T1/2 of ramiprilat, which is approximately 4–5 days. This final phase is due to the slow release of ramiprilat from its strong binding to ACE receptors. Despite the long final phase with a single oral dose of ramipril during the day at a dose of 2.5 mg or more, Css of ramiprilat is achieved after approximately 4 days of treatment. With a course prescription of the drug, the effective T1/2 (depending on the dose) is 13–17 hours.
Plasma protein binding is approximately 73% for ramipril and 56% for ramiprilat.
After intravenous administration, Vd of ramipril and ramiprilat are approximately 90 and 500 l, respectively.
After oral administration of radiolabeled ramipril (10 mg), 39% of the radioactivity is excreted through the intestines and about 60% by the kidneys. After intravenous administration of ramipril, 50–60% of the dose is found in the urine in the form of ramipril and its metabolites. After IV administration of ramiprilat, about 70% of the dose is found in the urine in the form of ramiprilat and its metabolites, in other words, with IV administration of ramipril and ramiprilat, a significant part of the dose is excreted through the intestines with bile, bypassing the kidneys (50 and 30%, respectively) . After oral administration of 5 mg ramipril in patients with bile duct drainage, almost equal amounts of ramipril and its metabolites are excreted by the kidneys and intestines during the first 24 hours after administration.
Approximately 80–90% of the metabolites in urine and bile were identified as ramiprilat and ramiprilat metabolites. Ramipril glucuronide and ramipril diketopiperazine account for approximately 10–20% of the total amount, and the level of unmetabolized ramipril in urine is approximately 2%.
In case of impaired renal function with creatinine Cl less than 60 ml/min, the excretion of ramiprilat and its metabolites by the kidneys slows down. This leads to an increase in the plasma concentration of ramiprilat, which decreases more slowly than in patients with normal renal function. When taking ramipril in high doses (10 mg), impaired liver function leads to a slower first-pass metabolism of ramipril to active ramiprilat and a slower elimination of ramiprilat. In healthy volunteers and patients with hypertension, after 2 weeks of treatment with ramipril at a daily dose of 5 mg, no clinically significant accumulation of ramipril and ramiprilat was observed. In patients with CHF, after 2 weeks of treatment with ramipril at a daily dose of 5 mg, an increase of 1.5–1.8 times in plasma ramiprilat concentrations and AUC was observed.
In healthy elderly volunteers (65–76 years), the pharmacokinetics of ramipril and ramiprilat do not differ significantly from those in young healthy volunteers.
Instructions for use of Adepress (Method and dosage)
The tablets are taken orally, without chewing, during meals, in the morning, 1 time per day. For the first two weeks, the dose is selected and adjusted individually - increased by 10 mg weekly until the treatment is effective. The daily dose cannot exceed 60 mg, and for elderly patients 40 mg.
The effect of treatment develops gradually. To prevent relapses, Adepress is prescribed in a maintenance dose for up to 4-6 months or more.
The instructions for use of Adepress contain a warning that if the drug is abruptly discontinued, withdrawal syndrome may develop, and therefore, discontinuation of use is carried out gradually. , suicidal tendencies may appear .
In elderly people, hyponatremia during drug therapy.
Contraindications
Amlodipine
hypersensitivity to amlodipine and other dihydropyridine derivatives;
severe arterial hypotension (SBP less than 90 mm Hg), shock (including cardiogenic);
an obstructive process that impedes the ejection of blood from the left ventricle (for example, clinically significant aortic stenosis);
hemodynamically unstable heart failure after myocardial infarction;
pregnancy;
breastfeeding period;
age under 18 years (safety and effectiveness have not been determined).
Ramipril
hypersensitivity to ramipril, other ACE inhibitors;
history of angioedema (hereditary or idiopathic, and also associated with previous therapy with ACE inhibitors);
hemodynamically significant stenosis of the renal arteries (bilateral or unilateral, in the case of a solitary kidney);
arterial hypotension (SBP less than 90 mm Hg) or conditions with unstable hemodynamic parameters;
hemodynamically significant stenosis of the aortic or mitral valve or hypertrophic obstructive cardiomyopathy;
primary hyperaldosteronism;
severe renal failure (Cl creatinine <20 ml/min/1.73 m2);
hemodialysis (experience of clinical use is insufficient);
nephropathy, which is treated with corticosteroids, NSAIDs, immunomodulators and/or other cytotoxic agents (experience of clinical use is insufficient);
decompensated chronic heart failure (experience of clinical use is insufficient);
hemodialysis or hemofiltration using certain types of membranes with a negatively charged surface, such as high-flow polyacrylonitrile membranes (risk of hypersensitivity reactions);
apheresis of LDL using dextran sulfate (risk of developing hypersensitivity reactions);
desensitizing therapy for hypersensitivity reactions to insect poisons - bees, wasps;
acute stage of myocardial infarction in patients with diseases such as severe heart failure (NYHA functional class IV); life-threatening ventricular arrhythmias; pulmonary heart;
simultaneous use of drugs containing aliskiren in patients with impaired renal function (Cl creatinine less than 60 ml/min) and patients with diabetes mellitus;
pregnancy;
breastfeeding period;
age under 18 years (experience of clinical use is insufficient).
Amlodipine + ramipril
hypersensitivity to the excipients included in the drug;
renal failure (Cl creatinine <20 ml/min/1.73 m2);
pregnancy;
breastfeeding period;
age under 18 years (experience of clinical use is insufficient).
With caution for the combination of amlodipine + ramipril: atherosclerotic lesions of the coronary and cerebral arteries (risk of excessive reduction in blood pressure); increased activity of the RAAS, in which, with ACE inhibition, there is a risk of a sharp decrease in blood pressure with deterioration of renal function; severe, especially malignant hypertension; CHF, especially severe or for which other drugs with antihypertensive effects are being taken; hemodynamically significant unilateral renal artery stenosis (in the presence of both kidneys); previous use of diuretics; disturbances in water and electrolyte balance, decrease in blood volume (including while taking diuretics, a salt-free diet, diarrhea, vomiting, profuse sweating); simultaneous use with drugs containing aliskiren (double blockade of the RAAS increases the risk of a sharp decrease in blood pressure, hyperkalemia and deterioration of renal function); liver function disorders (lack of experience with use: it is possible to either enhance or weaken the effects of ramipril; in patients with cirrhosis of the liver with ascites and edema, significant activation of the RAAS is possible); impaired renal function (creatinine Cl more than 20 ml/min); condition after kidney transplantation; systemic connective tissue diseases, incl. systemic lupus erythematosus, scleroderma, concomitant therapy with drugs that can cause changes in the peripheral blood picture (including allopurinol, procainamide) - possible inhibition of bone marrow hematopoiesis, development of neutropenia or agranulocytosis; diabetes mellitus (risk of developing hyperkalemia); old age (risk of increased antihypertensive effect); hyperkalemia; hyponatremia; CHF of non-ischemic etiology, functional class III–IV according to the NYHA classification; aortic stenosis; sick sinus syndrome; mitral stenosis; arterial hypotension; the only functioning kidney; renovascular hypertension; simultaneous use of dantrolene, estramustine, potassium-sparing diuretics and potassium preparations, potassium-containing substitutes for table salt, lithium preparations; surgery/general anesthesia; hemodialysis using high-flow membranes (for example AN69®).
Indications for use
- all types of depressive conditions, including those caused by alcohol addiction;
- panic disorders (episodic);
- bipolar affective disorders;
- dysthymia;
- social phobia;
- obsessive states;
- anxiety-phobic disorders;
- anorexia nervosa and bulimia ;
- post-traumatic stress disorders.
Use during pregnancy and breastfeeding
The drug is contraindicated for use, because ramipril may have an adverse effect on the fetus - impaired development of the fetal kidneys, decreased blood pressure in the fetus and newborns, impaired renal function, hyperkalemia, hypoplasia of the skull bones, oligohydramnios, contracture of the limbs, deformation of the skull bones, pulmonary hypoplasia. Before starting to take the drug in women of childbearing age, pregnancy should be excluded.
If a woman is planning a pregnancy, treatment with the drug should be discontinued. If pregnancy occurs during treatment with the drug, you should stop taking it as soon as possible and transfer the patient to take other drugs, the use of which will have the least risk for the child.
If treatment with the drug is necessary during breastfeeding, it should be discontinued (there are no data on the excretion of amlodipine and ramipril in women's breast milk).
Fertility
Amlodipine. Some patients receiving CCBs experienced reversible biochemical changes in the sperm heads. Clinical data are insufficient to assess the potential effect of amlodipine on fertility.
Side effects
The following undesirable effects are given in accordance with the following gradations of the frequency of their occurrence according to the WHO classification: very often - more than 1/10 (more than 10%); often - more than 1/100, but less than 1/10 (more than 1%, but less than 10%); uncommon - more than 1/1000, but less than 1/100 (more than 0.1%, but less than 1%); rarely - more than 1/10000, but less than 1/1000 (more than 0.01%, but less than 0.1%); very rarely - less than 1/10000 (less than 0.01%).
Amlodipine
From the cardiovascular system: often - peripheral edema (ankles and feet), palpitations; uncommon - excessive decrease in blood pressure, orthostatic hypotension, vasculitis; rarely - development or worsening of heart failure; very rarely - cardiac arrhythmias (including bradycardia, ventricular tachycardia and atrial fibrillation), myocardial infarction, chest pain, migraine.
From the musculoskeletal system and connective tissue: infrequently - arthralgia, muscle cramps, myalgia, back pain, arthrosis; rarely - myasthenia.
From the central nervous system and peripheral nervous system: often - a feeling of heat and a rush of blood to the skin of the face, increased fatigue, dizziness, headache, drowsiness; uncommon - malaise, fainting, increased sweating, asthenia, hypoesthesia, paresthesia, peripheral neuropathy, tremor, insomnia, mood lability, unusual dreams, nervousness, depression, anxiety; rarely - convulsions, apathy; very rarely - ataxia, amnesia, isolated cases of extrapyramidal syndrome have been reported.
From the digestive system: often - abdominal pain, nausea; uncommon - vomiting, changes in bowel habits (including constipation, flatulence), dyspepsia, diarrhea, anorexia, dry oral mucosa, thirst; rarely - gum hyperplasia, increased appetite; very rarely - gastritis, pancreatitis, hyperbilirubinemia, jaundice (usually cholestatic), increased activity of liver transaminases, hepatitis.
Blood disorders: very rarely - thrombocytopenic purpura, thrombocytopenia, leukopenia.
Metabolic disorders: very rarely - hyperglycemia.
From the respiratory system: infrequently - shortness of breath, rhinitis; very rarely - cough.
From the kidneys and urinary tract: infrequently - frequent urination, painful urination, nocturia, impotence; very rarely - dysuria, polyuria.
Allergic reactions: infrequently - skin itching, rash; very rarely - angioedema, erythema multiforme, urticaria.
Other: infrequently - alopecia, tinnitus, gynecomastia, weight gain/loss, blurred vision, diplopia, accommodation disturbance, xerophthalmia, conjunctivitis, eye pain, taste disturbance, chills, nosebleeds; rarely - dermatitis; very rarely - parosmia, xeroderma, cold sweat, skin pigmentation disorder.
Ramipril
From the heart: uncommon - myocardial ischemia, including the development of an attack of angina or MI, tachycardia, arrhythmias (appearance or intensification), palpitations, peripheral edema.
From the side of blood vessels: often - excessive decrease in blood pressure, disturbance of orthostatic regulation of vascular tone (orthostatic hypotension), syncope; infrequently - a rush of blood to the skin of the face; rarely - the occurrence or intensification of circulatory disorders against the background of stenotic vascular lesions, vasculitis; frequency unknown - Raynaud's syndrome.
From the side of the central nervous system: often - headache, feeling of lightness in the head; uncommon - dizziness, ageusia (loss of taste sensitivity), dysgeusia (impaired taste sensitivity), paresthesia (burning sensation); rarely - tremor, imbalance; frequency unknown - cerebral ischemia, including ischemic stroke and transient cerebrovascular accident, impaired psychomotor reactions, parosmia (impaired odor perception).
From the organ of vision: infrequently - visual disturbances, including blurred visual perception; rarely - conjunctivitis.
On the part of the hearing organ: rarely - hearing impairment, ringing in the ears.
From the psyche: infrequently - depressed mood, anxiety, nervousness, restlessness, sleep disturbances, including drowsiness; rarely - confusion; frequency unknown - impaired concentration.
From the respiratory system: often - dry cough (worsening at night and when lying down), bronchitis, sinusitis, shortness of breath; infrequently - bronchospasm, including worsening of bronchial asthma, nasal congestion.
From the digestive system: often - inflammatory reactions in the stomach and intestines, digestive disorders, discomfort in the abdominal area, dyspepsia, diarrhea, nausea, vomiting; uncommon - pancreatitis, incl. and with a fatal outcome (cases of pancreatitis with a fatal outcome when taking ACE inhibitors were observed extremely rarely), increased activity of pancreatic enzymes in the blood plasma, intestinal angioedema, abdominal pain, gastritis, constipation, dry oral mucosa; rarely - glossitis; frequency unknown - aphthous stomatitis (inflammatory reaction of the oral mucosa).
From the hepatobiliary system: infrequently - increased activity of liver enzymes and the content of conjugated bilirubin in the blood plasma; rarely - cholestatic jaundice, hepatocellular lesions; frequency unknown - acute liver failure, cholestatic or cytolytic hepatitis (death was extremely rare).
From the kidneys and urinary tract: rarely - impaired renal function, including the development of acute renal failure, increased urine output, increased pre-existing proteinuria, increased concentrations of urea and creatinine in the blood.
From the reproductive system and mammary glands: infrequently - transient impotence due to erectile dysfunction, decreased libido; frequency unknown - gynecomastia.
From the blood and lymphatic system: infrequently - eosinophilia; rarely - leukopenia, including neutropenia and agranulocytosis, a decrease in the number of red blood cells in the peripheral blood, a decrease in hemoglobin, thrombocytopenia; frequency unknown - suppression of bone marrow hematopoiesis, pancytopenia, hemolytic anemia.
From the skin and mucous membranes: often - skin rash, in particular maculopapular; uncommon - angioedema, incl. and with a fatal outcome (swelling of the larynx can cause obstruction of the airways, leading to death), itching, hyperhidrosis (increased sweating); rarely - exfoliative dermatitis, urticaria, onycholysis; very rarely - photosensitivity reactions; frequency unknown - toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme, pemphigus, worsening psoriasis, psoriasis-like dermatitis, pemphigoid or lichenoid (lichenoid) exanthema or enanthema, alopecia.
From the musculoskeletal system and connective tissue: often - muscle cramps, myalgia; infrequently - arthralgia.
From the side of metabolism, nutrition and laboratory parameters: often - increased potassium content in the blood; infrequently - anorexia, loss of appetite; frequency unknown - decreased sodium concentration in the blood, syndrome of inappropriate ADH secretion.
From the immune system: frequency unknown - anaphylactic or anaphylactoid reactions (with ACE inhibition, the number of anaphylactic or anaphylactoid reactions to insect venoms increases), increased titer of antinuclear antibodies.
General disorders: often - chest pain, feeling tired; uncommon - increased body temperature; rarely - asthenia (weakness).
Interaction
Amlodipine
It can be expected that inhibitors of liver microsomal oxidation enzymes (erythromycin in young people, diltiazem in elderly people, ketoconazole, itraconazole, ritonavir) will increase the concentration of amlodipine in the blood plasma, increasing the risk of side effects, and inducers of liver microsomal oxidation enzymes will decrease it. With simultaneous use of amlodipine with cimetidine, the pharmacokinetics of amlodipine does not change.
A simultaneous single dose of 240 ml of grapefruit juice and 10 mg of amlodipine orally is not accompanied by a significant change in the pharmacokinetics of amlodipine. Unlike other CCBs, no clinically significant interaction was found with amlodipine (III generation CCB) when used together with NSAIDs, especially indomethacin.
It is possible to enhance the antianginal and antihypertensive effect of CCBs when used together with thiazide and loop diuretics, verapamil, ACE inhibitors, beta-blockers and nitrates, as well as increase their antihypertensive effect when used together with a