The role of the drug Bilobil in the treatment of patients with vascular diseases of the brain
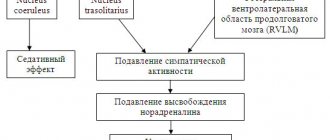
Patients with mild cognitive impairment may experience: • a moderate decrease in the ability to concentrate; • deterioration of simultaneous perception; • deterioration of memory for current events, the names of new acquaintances and geographical names; • absent-mindedness, frequent search for forgotten things; • increased fatigue during mental work; • decreased mood and anxiety due to awareness of one’s own inferiority; • moderate cognitive impairment does not lead to loss of ability to work or to a significant reduction in normal household and social activity. A pronounced degree of cognitive impairment - dementia is characterized by impairments of memory and intelligence, leading to varying degrees of social maladjustment. According to epidemiological data, 4–6% of patients who have had a stroke subsequently develop dementia within a year. The most common cause of cognitive impairment, especially in elderly patients, is chronic vascular diseases of the brain (CVCD), better known in our country under the term dyscirculatory encephalopathy. Discirculatory encephalopathy is heterogeneous in its etiology. The following main types of dyscirculatory encephalopathy are distinguished: • hypertensive encephalopathy (subcortical arteriosclerotic encephalopathy and hypertensive multi-infarct encephalopathy); • atherosclerotic encephalopathy; • chronic vascular vertebrobasilar insufficiency; • mixed forms. Cognitive impairment in discirculatory encephalopathy progresses gradually or stepwise, reaching the final stage (on average after 10–15 years) to the degree of dementia. Despite the progressive process of increase in cognitive impairment, periods of stabilization and even periods of improvement are possible. Cognitive disorders in dyscirculatory encephalopathy are associated with predominant damage to the periventricular substance of the frontal lobes, where the so-called “cognitive pathways” (frontothalamic, frontolimbic) pass. The most common cognitive impairments occur in subcortical arteriosclerotic encephalopathy and hypertensive multi-infarct encephalopathy. In hypertensive multi-infarct encephalopathy, the development of cognitive impairment is associated with the location of the infarction in functionally significant areas of the brain. Most often, cognitive impairment occurs when lacunar infarctions are localized in the anteromedial parts of the thalamus and in the area of the frontothalamic tract. In addition to cognitive impairment, patients with CSHM and patients in the recovery and residual post-stroke periods are characterized by emotional and volitional disorders (depression, asthenia, anxiety), headaches and dizziness, gait disturbances (associated in patients who have suffered a stroke with leg paresis or ataxia; in patients with CSHM – with frontal gait dyspraxia). The features of drug therapy for patients with chronic hepatitis B and the consequences of stroke include: • the need to prescribe, along with etiopathogenetic therapy, a larger number of drugs with syndromological action; • a large percentage of complications and side effects in patients with chronic hepatitis B, which arise during treatment and cause distrust in patients to “chemical” drugs. To a certain extent, this can be avoided by using herbal preparations prepared on the basis of ginkgo biloba, which have a multimodal effect and practically no complications. These drugs include Bilobil and Bilobil forte, one capsule of which contains 80 mg of ginkgo biloba extract. The main active ingredients of Bilobil and Bilobil forte are flavonoid glycosides, terpene substances (ginkgolides A, B, C, bilobalide) and proanthocyanidins, which have a positive effect on the processes of free radical oxidation, tissue metabolism and microcirculation. Under experimental conditions, it has been shown that ginkgo biloba affects neurotransmitter processes in the central nervous system by enhancing the release of neurotransmitters from presynaptic nerve terminals, inhibiting the reuptake of biogenic amines and increasing the “sensitivity” of postsynaptic muscarinic receptors to acetylcholine. In our country, the first major study of the effectiveness of the drug ginkgo biloba was conducted in 1998 by N.N. Yakhno et al. in patients with early forms of chronic disorders of cerebral blood flow. The therapy has been shown to have a positive effect on memory, concentration and attention span, associative processes, and psychomotor functions. The effect of therapy was quite persistent, as evidenced by follow-up observation of some patients [Yakhno N.N. et al., 2005]. Later it was found that Bilobil not only improves cerebral circulation and the supply of oxygen and glucose to the brain, but also prevents red blood cell aggregation, inhibits platelet activating factor, stimulates the production of endothelium-dependent relaxing factor, dilates small arteries, increases the tone of veins, thereby regulating blood filling of blood vessels . A large number of studies have been conducted to study the effectiveness of ginkgo biloba preparations in various diseases accompanied by cognitive impairment [Zakharov V.V., Yakhno N.N., 2004; Birks J. et al., 2007; Mix JA et al., 2002]. The vast majority of these studies found a positive effect of ginkgo biloba on cognitive function. Considering that dementia progresses over time, stabilization of the condition during the use of ginkgo biloba may indicate its undeniable effectiveness. The greatest effect of ginkgo biloba is observed in mild neurological disorders, which indicates the advisability of the earliest use of this drug in dyscirculatory encephalopathy [Solomon P., 2002]. A study conducted by V.A. Parfenov and Yu.A. Starchina (2005), M.M. Equally, A.Yu. Emelin (2006), showed that in patients with BMS, the use of drugs containing ginkgo biloba improves cognitive functions, reduces the severity of headaches, dizziness and emotional disorders. Summarizing the results of many clinical studies, it can be argued that Bilobil has the following effects: • has a positive effect on metabolic processes in the central nervous system, improving the state of cognitive functions and the emotional-volitional sphere; • improves cerebral circulation by increasing the elastic properties of the vascular wall; • reduces the aggregation ability of erythrocytes and platelets, thereby preventing the development and progression of cerebral ischemia; • improves the condition of the microcirculatory bed. Bilobil is indicated for the following patients: • patients who have suffered an ischemic stroke, in order to improve the state of cognitive functions, emotional-volitional sphere, reduce the severity of headaches, dizziness, ataxic syndrome, etc.; • patients with chronic hepatitis B in order to slow the progression of the disease, improve cognitive functions and emotional-volitional sphere, improve walking functions; • patients with SHM and concomitant peripheral circulatory disorders (atherosclerotic damage to the vessels of the heart, legs and renal arteries), diabetic angioretinopathy, etc. Standardized ginkgo extract preparations are taken 80 mg 2-3 times a day, usually during or after meals, with water. Patients with difficulty falling asleep should not take ginkgo biloba preparations immediately before bedtime; the evening dose should be no later than 18–19 hours. The course of treatment is from 4 to 12 weeks, in some cases longer treatment is required. It is recommended to conduct 2-3 courses throughout the year. Bilobil is well tolerated, side effects are rare (1–2% of cases) and are usually represented by harmless dyspeptic symptoms, headache, nervousness, sleep disturbances, and skin allergic reactions. Literature 1. Zakharov V.V., Yakhno N.N. Syndrome of mild cognitive impairment in old and senile age. // RMJ – 2004 – No. 10 – pp. 573–576. 2. Odinak M.M., Emelin A.Yu. Experience of using Tanakan in the treatment of moderate cognitive disorders. // RMJ – 2006 – T. 14 – No. 23 – P. 1681–1686. 3. Parfenov V.A., Starchina Yu.A. Tanakan treatment of neurological disorders in patients with arterial hypertension. // RMJ – 2005 – T. 13 – No. 22 – P. 1462–1465. 4. Preobrazhenskaya I.S., Yakhno N.N. Vascular cognitive disorders - clinical manifestations, diagnosis, treatment. // Neurological Journal – 2007 – T. 12 – P. 45–51. 5. Yakhno N.N., Zakharov V.V., Lokshina A.B. Syndrome of mild cognitive impairment in dyscirculatory encephalopathy. // Journal of Neurology and Psychiatry – 2005 – No. 2 – pp. 13–17. 6. Yakhno N.N., Damulin I.V., Zakharov V.V., Elkin M.N., Erokhina L.G., Stakhovskaya L.V., Chekneva N.S., Suslina Z. A.A., Timerbaeva S.L., Fedin P.A., Bodareva E.A., Skoromets A.A., So¬ro¬koumov V.A., Ivashkin V.T., Gigoriev Yu.V. ., Pervozvansky B.E. Use of Tanakan in the initial stages of cerebrovascular insufficiency: results of an open multicenter study. // Neurological Journal – 1998 – T. 3 – pp. 18–22. 7. Birks J., Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Review. // Cochrane Database Syst. Rev. – 2007 – Vol. 18, N 2 – P. 124–132. 8. Bowler JV Vascular cognitive impairment. // Stroke – 2004 – V. 35, N 2 – P. 386–388. 9. Brandt J. Mild cognitive impairment in the elderly. Review // Am. Fam. Physician – 2001 – Vol. 63; N 4 – P. 620–626 10. Christen Y. Ginkgo biloba and neurodegenerative disorders. Review // Front. Biosci. – 2004 – Vol. 1; N 9 – P. 3091–3104. 11. Mix JA, Crews WD A double–blind, placebo–controlled, randomized trial of Ginkgo biloba extract EGb 761 in a sample of cognitively intact older adults: neuropsychological fmdings. //Hum.Psychopharmacol. – 2002 – Vol. 17; N 6 – P. 267–277. 12. Solomon P., Adams F., Silver A. et al. Ginkgo for Memory Enhancement. A randomized. Controlled. trial. // JAMA – 2002 –N. 288 – P. 835–840.
Instructions for use BILOBIL® INTENSE
Before starting to use Bilobil® intense capsules, you must make sure that the symptoms presented in the “Indications” section are not the result of another underlying disease requiring specific treatment.
The drug is not recommended for use by patients with a tendency to bleeding or disorders of the blood coagulation system. Patients with an increased risk of bleeding (hemorrhagic diathesis), as well as patients taking anticoagulant and antiplatelet drugs, should consult a doctor before taking the drug.
Medicines containing ginkgo biloba may increase the risk of bleeding. The drug should be stopped 3-4 days before surgery.
In patients with epilepsy, taking medications containing ginkgo biloba may trigger the development of a seizure.
Due to the lactose content, the drug is not recommended for patients with congenital galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome.
If symptoms persist or worsen while taking the drug, consult a doctor.
Impact on the ability to drive vehicles and operate machinery
No studies have been conducted.
Preclinical safety data
Chronic toxicity.
Chronic toxicity was studied in rats and dogs when administered orally for 6 months at daily doses of 20 and 100 mg/kg body weight (corresponding to safety factors of up to 3.3 in rats and 11.6 in dogs), as well as when administered at doses of 300, 400 and 500 mg/kg body weight in rats or 300, 400 mg/kg body weight in dogs (which corresponds to safety factors of up to 16.8 in rats and 46.3 in dogs). The results showed low toxicity only in dogs at the highest doses.
Reproductive toxicity.
Data are limited only to information on the reproductive toxicity of Ginkgo biloba dry extract. Published data are contradictory. While older studies in rats, rabbits, and newer studies in mice did not show teratogenic, embryotoxic, or adverse reproductive effects, one study in mice showed effects on reproductive outcomes such as fertility and reproductive output, as well as the development of vaginal bleeding. Also, studies with various extracts of Ginkgo biloba have revealed effects on fetal development (with and without maternal toxicity) or have found the development of subcutaneous hemorrhages, hypopigmentation, growth inhibition and anophthalmia in chick embryos. There are no adequate reproductive toxicity studies.
Mutagenicity, carcinogenicity.
Genotoxicity and carcinogenicity studies are not available for Ginkgo biloba dry extract. An extract similar to the standardized extract of Ginkgo biloba has been studied in a number of studies for genotoxicity and carcinogenicity. The bacteria tested positive for gene mutations. Micronucleus testing of mouse peripheral blood erythrocytes was negative in males and equivocal in females. Carcinogenicity studies in rats found thyroid tumors, and hepatocellular carcinomas were found in carcinogenicity studies in mice. These changes are considered specific to rodents. These types of tumors are not considered relevant to humans. The extract did not cause measurable genotoxic effects when administered to mice at doses up to 2000 mg/kg.