Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2022 Pharmacy 84.
Sodium chloride
When performing any infusion, it is necessary to monitor the patient's condition, clinical and biological indicators, it is especially important to evaluate plasma electrolytes. In children, sodium excretion may slow down due to immature kidney function. Therefore, in such patients, repeated infusions should be carried out only after determining the plasma sodium concentration.
Hypersensitivity reactions
There is evidence of the development of hypersensitivity reactions or infusion reactions during the use of the drug, including hypotension, pyrexia, tremor, chills, urticaria, rash and itching. If hypersensitivity reactions or infusion reactions occur, the infusion should be stopped immediately and the necessary therapeutic measures taken as indicated.
Risk of hypervolemia and/or solute overload and electrolyte imbalances
Depending on the volume and speed of infusion, the following conditions may develop during intravenous administration of the drug:
- hypervolemia and (or) solute overload, leading to overhydration and, for example, congestion, including central and peripheral edema;
-clinically significant disturbances in electrolyte and acid-base balance.
Use in patients with renal failure
In patients with renal failure, the drug should be used with extreme caution or not used at all. Use of the drug in such patients may lead to sodium retention.
Use only a clear solution, without visible inclusions and if the packaging is not damaged. Administer immediately after connecting to the infusion system. The solution should be administered using sterile equipment in compliance with the rules of asepsis and antisepsis. To prevent air from entering the infusion system, it should be filled with solution.
As with all parenteral solutions, the compatibility of added substances with the solution must be determined before reconstitution.
Drugs known to be incompatible with it should not be used with sodium chloride solution 0.9%. The compatibility of added medicinal substances with a 0.9% sodium chloride solution should be determined by a doctor by checking for possible changes in color and/or the appearance of sediment, insoluble complexes or crystals. Before addition, it is necessary to determine whether the substance being added is soluble and stable in water at the pH level of a 0.9% sodium chloride solution. When adding a drug, it is necessary to determine the isotonicity of the resulting solution before administration. Before adding drugs to the solution, they must be thoroughly mixed in compliance with aseptic rules.
The prepared solution should be administered immediately after preparation, do not store!
Adding other drugs or changing the administration technique may cause fever due to the possible entry of pyrogens into the body. In case of development of undesirable reactions, it is necessary to immediately stop administering the solution.
In case of significant dehydration, if intravenous administration is not possible, the drug is administered subcutaneously or rectally.
Sodium chloride is the most important chemical reagent
Sodium chloride forms the basis of table salt, the most popular food seasoning. In addition, millions of tons of sodium salt of hydrochloric acid are consumed by industry for various purposes.
Properties of sodium chloride
NaCl is a crystal with a cubic ionic lattice. Crystals are colorless and odorless, with a distinct salty taste. Sodium chloride is water soluble and not hygroscopic. Dissolves in ammonia, ethyl and methyl alcohol, formic acid, glycerin, ethylene glycol. Insoluble in acetone, hydrochloric acid, diethyl ether.
An aqueous solution of sodium chloride freezes at temperatures below zero. Thanks to this property, the chemical reagent was used for quite a long time as a de-icing agent on roads and sidewalks (now this method is considered environmentally harmful and is used less and less often). The use of a mixture of finely crushed ice and table salt as an effective and simple coolant that can lower the temperature of the mixture by a couple of tens of degrees is based on this same property.
Sodium chloride in nature and human life
NaCl is very important for humans: we need it to absorb nutrients during digestion; our blood consists to a large extent of saline solution; Sodium chloride is one of the most important sources of mineral raw materials for the chemical industry.
In nature, sodium chloride is found in sea water, salt lakes, saline groundwater, and in fossil form (halite, rock salt). Halite is the mineral form of NaCl. Halite is sometimes called rock salt, but this is incorrect. Rock salt is a sedimentary rock containing 90% halite and admixtures of iron, gypsum, magnesite, talc, bitumen, compounds of potassium, calcium, magnesium, iodine, bromine, boron. Each rock salt deposit has its own unique composition.
Sea salt, obtained by evaporating sea water, contains more impurities of chemical elements, minerals and salts that are vital for humans than rock salt. It contains only 77.8% sodium chloride. It has a bitter taste and is used mainly in medicine and cooking.
Application of NaCl
— The chemical reagent is used to produce more than one and a half thousand substances and materials, ranging from metallic sodium and chlorine to soda, hydrochloric acid, sodium hydroxide, and herbicides for agriculture.
— Table salt is a finished product consisting of 97 percent or more NaCl, used in the food industry. The higher the sodium chloride content, the higher the grade of salt. Specialized varieties with added microelements, for example, iodized salt, are also produced. Table salt is used as a seasoning in food and as a preservative.
— In industrial and semi-industrial water treatment for the regeneration of ion exchange resins in sodium cation exchange filters.
— Solutions of sodium chloride are in demand in medicine for the preparation and dilution of medicines, to restore the body’s water-salt balance, for bleeding, as an antidote for silver nitrate poisoning, as an antibacterial agent for treating wounds, and for some other purposes.
- In zoology - for addition to the diet of animals.
The Prime Chemicals Group store offers to buy table salt wholesale and retail at a good price. We also have a wide range of other reagents for laboratory research and industry, laboratory glassware and instruments.
Hydrolysis
Hydrolysis (Greek hydor - water and lysis - destruction) is the process of splitting molecules of complex chemical substances due to reaction with water molecules.
In chemistry, as in life, it is most often the unstable and weak that are destroyed (the persistent and strong can withstand blows). Remember that hydrolysis (water) destroys the “weak” - this rule will be very useful to you.
Any salt consists of a base and an acid. Absolutely any:
- NaCl is a derivative of the base NaOH and the acid HCl
- KNO3 is a derivative of the base KOH and the acid HNO3
- CuSO4 is a derivative of the base Cu(OH)2 and the acid H2SO4
- Al3PO4 is a derivative of the base Al(OH)3 and the acid H3PO4
- Ca(NO2)2 is a derivative of the base Ca(OH)2 and the acid HNO2
To successfully solve problems on the topic of hydrolysis and write reactions, you should remember which bases and acids are weak and which are strong.
When studying hydrolysis, I recommend that students save the diagram you see below on their gadget. In order to gain the necessary experience, it is irreplaceable. Use it as often as possible, peek into it and it will quietly appear in your intellectual component 
By cation, by anion, or no hydrolysis?
So, if the salt contains a strong base residue and a strong acid residue, hydrolysis does not occur. Examples: NaCl, KBr, CaSO4. Also, hydrolysis does not occur if the salt is insoluble (regardless of what it is formed by): AlPO4, FeSO3, CaSO3.
If the salt contains a residue of a weak base and a residue of a strong acid, then hydrolysis occurs along the cation. Remember that hydrolysis destroys the weak, in this case the cation. Examples: AlCl3, MgBr2, Cr2SO4, NH4NO3.
The NH4+ cation and its base NH4OH, despite the solubility, are weak, so hydrolysis will proceed through the cation into the NH4Cl salt. I also note that Ca(OH)2 is considered a soluble base, so hydrolysis of the CaCl2 salt does not occur.
If the salt contains a strong base residue and a weak acid residue, then hydrolysis occurs along the anion. Examples: K3PO4, NaNO2, Ca(OCl)2, Ba(CH3COO)2, Li2SiO3.
If a salt is formed by the remainder of a weak base and a weak acid, then hydrolysis occurs at both the cation and the anion. Examples: Mg(NO2)2, Al2S3, Cr2(SO3)3, CH3COONH4.
Independently determine the type of hydrolysis for CaI2, Li2SiO3, Ba(NO2)2, CuBr2, Zn(H2PO4)2. Below you will find the solution.
Solution medium
The solution medium can be neutral, acidic or alkaline. Determined by the type of hydrolysis. Some tasks may be structured so that when you see salt, you will have to determine its type of solution.
Let me make you happy: if you have mastered the topic of hydrolysis, doing it is as easy as shelling pears. In the case when hydrolysis does not occur or occurs at both the cation and the anion, the solution medium is neutral.
If hydrolysis occurs along the cation (the base residue is destroyed), the medium is acidic; if hydrolysis occurs along the anion (the acid residue is destroyed), then the solution medium will be alkaline. Study the examples.
However, I note that in dihydrogen phosphates, hydrosulfites and hydrosulfates, the environment is always acidic due to the characteristics of dissociation. Examples: NH4H2PO4, LiHSO4. In hydrophosphates, the environment is alkaline due to the fact that the dissociation constant for the third step is less than the hydrolysis constant. Examples: K2HPO4, Na2HPO4.
Try to identify the solution environment for the compounds from the independent exercise you just completed. The solution will be located below.
In order to confuse tasks, synonyms are often given. So the “solution medium” can be replaced by the pH value.
Remember that an acidic environment has a pH of 7.
For example, in CaCl2 salt the solution environment will be neutral (pH = 7), and in AlCl3 solution it will be acidic (pH
Indicators (lat. indicator - pointer)
An indicator is a substance used in chemistry to determine the environment of a solution. Depending on the environment of the solution, the indicator can change its color, which clearly reflects the nature of the environment at a certain point in time.
The most well-known and widely used indicators are litmus, phenolphthalein and methyl orange. Depending on the solution environment, their color changes, as reflected in the table below.
For those who have a good visual memory, it will not be difficult to remember this diagram. But what about auditory and kinesthetic learners? Due to exam anxiety, such a table can easily dissolve and get confused in the ocean of thoughts, so I recommend that my students memorize the indicators by verse.
But what about auditory and kinesthetic learners? Due to exam anxiety, such a table can easily dissolve and get confused in the ocean of thoughts, so I recommend that my students memorize the indicators by verse.
Just imagine how pleasant it will be to read a verse during an exam and be convinced of its infallibility. This will give you confidence and lift your spirits 
Litmus The red litmus indicator will clearly indicate the acid. Blue litmus indicator - The alkali is here, don’t be so open! When the environment is neutral, it is always purple.
Phenolphthalein Phenolphthalein In alkalis it is crimson. Despite this, in acids it is colorless.
Methyl orange From alkali I am yellow as in a fever I turn pink from acids as from shame And I throw myself into the water without looking back - Here I am almost always orange!
© Bellevich Yuri Sergeevich 2018-2021
This article was written by Yuri Sergeevich Bellevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without the prior consent of the copyright holder is punishable by law. To obtain article materials and permission to use them, please contact Yuri Bellevich
.
1.4.7. Hydrolysis of salts. Aqueous solution environment: acidic, neutral, alkaline.
In order to understand what hydrolysis of salts is, let us first remember how acids and alkalis dissociate.
What all acids have in common is that their dissociation necessarily produces hydrogen cations (H+), while the dissociation of all alkalis always produces hydroxide ions (OH−).
In this regard, if in a solution, for one reason or another, there are more H+ ions, the solution is said to have an acidic reaction of the medium, if OH− - an alkaline reaction of the medium.
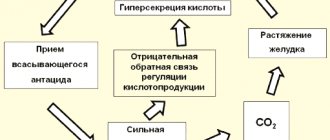
If everything is clear with acids and alkalis, then what reaction of the medium will be in salt solutions?
At first glance, it should always be neutral. And really, where, for example, in a sodium sulfide solution does the excess of hydrogen cations or hydroxide ions come from? Sodium sulfide itself upon dissociation does not form ions of one or another type:
Na2S = 2Na+ + S2-
However, if you were faced with, for example, aqueous solutions of sodium sulfide, sodium chloride, zinc nitrate and an electronic pH meter (a digital device for determining the acidity of a medium), you would find an unusual phenomenon. The device would show you that the pH of the sodium sulfide solution is greater than 7, i.e. there is a clear excess of hydroxide ions. The sodium chloride solution would be neutral (pH = 7), and the Zn(NO3)2 solution would be acidic.
The only thing that meets our expectations is the sodium chloride solution environment. She turned out to be neutral, as expected.
But where did the excess of hydroxide ions in a solution of sodium sulfide and hydrogen cations in a solution of zinc nitrate come from?
Let's try to figure it out. To do this, we need to understand the following theoretical points.
Any salt can be thought of as the product of the interaction of an acid and a base. Acids and bases are divided into strong and weak. Let us recall that those acids and bases whose degree of dissociation is close to 100% are called strong.
note: sulfur (H2SO3) and phosphoric (H3PO4) are often classified as medium-strength acids, but when considering hydrolysis tasks they should be classified as weak.
Acidic residues of weak acids are capable of reversibly interacting with water molecules, removing hydrogen cations H+ from them. For example, the sulfide ion, being the acidic residue of a weak hydrogen sulfide acid, interacts with it as follows:
S2- + H2O ↔ HS− + OH−
HS−+ H2O ↔ H2S + OH−
As you can see, as a result of this interaction, an excess of hydroxide ions is formed, which is responsible for the alkaline reaction of the medium. That is, the acidic residues of weak acids increase the alkalinity of the environment. In the case of salt solutions containing such acidic residues, they are said to experience hydrolysis at the anion.
Acidic residues of strong acids, unlike weak ones, do not interact with water. That is, they do not affect the pH of the aqueous solution. For example, the chloride ion, being the acidic residue of strong hydrochloric acid, does not react with water:
That is, chloride ions do not affect the pH of the solution.
Of the metal cations, only those that correspond to weak bases are able to interact with water. For example, the Zn2+ cation, which corresponds to the weak base zinc hydroxide. The following processes occur in aqueous solutions of zinc salts:
Zn2+ + H2O ↔ Zn(OH) + + H+
Zn(OH) + + H2O ↔ Zn(OH)+ + H+
As can be seen from the equations above, as a result of the interaction of zinc cations with water, hydrogen cations accumulate in the solution, increasing the acidity of the environment, that is, lowering the pH. If the salt contains cations that correspond to weak bases, then the salt is said to hydrolyze at the cation.
Metal cations, which correspond to strong bases, do not interact with water. For example, the Na+ cation corresponds to a strong base - sodium hydroxide. Therefore, sodium ions do not react with water and do not affect the pH of the solution in any way.
Thus, based on the above, salts can be divided into 4 types, namely those formed:
1) a strong base and a strong acid,
Such salts do not contain either acidic residues or metal cations that interact with water, i.e. capable of affecting the pH of an aqueous solution. Solutions of such salts have a neutral reaction environment. Such salts are said to not undergo hydrolysis .
Examples: Ba(NO3)2, KCl, Li2SO4, etc.
2) strong base and weak acid
In solutions of such salts, only acidic residues react with water. The medium of aqueous solutions of such salts is alkaline; in relation to salts of this type they say that they hydrolyze at the anion
Examples: NaF, K2CO3, Li2S, etc.
3) weak base and strong acid
In such salts, cations react with water, but acidic residues do not react - hydrolysis of the salt by cation , the medium is acidic.
Examples: Zn(NO3)2, Fe2(SO4)3, CuSO4, etc.
4) a weak base and a weak acid.
Both cations and anions of acidic residues react with water. Hydrolysis of salts of this kind occurs both at the cation and at the anion . Often such salts undergo irreversible hydrolysis .
What does it mean that they are irreversibly hydrolyzed?
Since in this case both metal cations (or NH4+) and anions of the acidic residue react with water, both H+ ions and OH− ions appear in the solution at the same time, which form an extremely poorly dissociating substance - water (H2O).
This, in turn, leads to the fact that salts formed by acidic residues of weak bases and weak acids cannot be obtained by exchange reactions, but only by solid-phase synthesis, or cannot be obtained at all. For example, when mixing a solution of aluminum nitrate with a solution of sodium sulfide, instead of the expected reaction:
2Al(NO3)3 + 3Na2S = Al2S3 + 6NaNO3 (− the reaction does not proceed this way!)
The following reaction is observed:
2Al(NO3)3 + 3Na2S + 6H2O= 2Al(OH)3↓+ 3H2S↑ + 6NaNO3
However, aluminum sulfide can be easily obtained by fusing aluminum powder with sulfur:
2Al + 3S = Al2S3
When aluminum sulfide is added to water, it, just like when trying to obtain it in an aqueous solution, undergoes irreversible hydrolysis.
Al2S3 + 6H2O = 2Al(OH)3↓ + 3H2S↑
Section: Theory for preparing for the Unified State Exam Author: S.I. Wide-belted
157 320
SODIUM CHLORIDE . Sodium chloridum.Synonyms: sodium chloride, table salt.
Properties.
White cubic crystals or white crystalline powder, odorless, salty taste, soluble in three parts of water (solubility at 20°C - 36.0; at 100°C - 39.1), slightly soluble in glycerin and methanol. Solutions are sterilized in an autoclave at a temperature of 120°C for 20 minutes.
Release form. For medicinal purposes, industrially produced tablets of 0.9 g or sodium chloride powder are used, from which sterile hypertonic solutions are prepared in distilled water at 5, 10 and 20% concentrations. It is also available in the form of a 0.9% sterile solution in ampoules of 5, 10 ml and bottles of 100, 200, 250, 400 ml.
Store under normal conditions, shelf life is unlimited.
Action and application.
Sodium chloride is the main source of formation of hydrochloric acid in gastric juice. When taken orally, it is well absorbed from the gastrointestinal tract; when administered intravenously, hypertonic solutions spread through the bloodstream, exerting a selective effect on organs and tissues, centers of water and salt metabolism.
Excess substances are excreted within 24 hours through the kidneys; the process of excretion of sodium chloride is closely related to the excretion of water and is regulated by hormones of the pituitary gland (antidiuretic hormone) and adrenal glands (aldosterone).
Depending on the concentration of sodium chloride, solutions are distinguished: isotonic (0.9%), hypertonic (1% or more) and hypotonic (less than 0.85%).
Hypertonic solutions of sodium chloride have a reflexive and resorptive effect on the body, stimulating receptors in the veins, heart, lungs and abdominal organs; affect water and mineral metabolism, osmotic and acid-base balance, motor and secretory function of the gastrointestinal tract, excretory function of the kidneys and the volume of diuresis; exhibit antitoxic effects; activate the body's immunobiological reactions. Their use as a means of pathogenetic and replacement therapy is based on these properties of hypertonic sodium chloride solutions.
The osmotic and diuretic effects of hypertonic sodium chloride solutions determine their antitoxic effect. In this case, there is a rapid replacement of sodium and chlorine and activation of sodium-containing enzymes, such as Na + and K + -adenosine triphosphatase, which are involved in the antitoxic reactions of the body. The complex system of tissue regulation of water-salt metabolism, the so-called sodium-potassium pump, is also stimulated, which promotes rapid rehydration of the body. Dehydration (dehydration), observed in gastrointestinal diseases of young animals of early age, is accompanied by significant losses of water, sodium and chlorine. This leads to life-threatening blood thickening, slowing of blood flow, metabolic disorders, hypoxia, and hypothermia. Restoring the water balance of blood and tissues - rehydration - is possible only with the rapid replacement of lost sodium and chlorine ions, which play an active role in water-salt metabolism. Sodium and chlorine pass actively through cell membranes, water follows them passively. Deficiency of sodium and chlorine is most quickly restored by intravenous administration of hypertonic solutions. Increasing the concentration of the solution to 3-5% increases the sodium chloride content in the interstitial fluid 10-20 times.
Hypertonic solutions of sodium chloride, when administered intravenously, increase the reserve alkalinity of the blood, which can be used as a means of alkalizing therapy for acidosis in pregnant cows. Regulation of acid-base balance in pregnant cows simultaneously leads to an increase in the activity of the body's immunobiological reactions (phagocytosis) and natural resistance in newborn calves.
Hypertonic solutions of sodium chloride, acting indirectly through parasympathetic endings, increase the formation of acetylcholine and stimulate the motor function of the gastrointestinal tract. This is the basis for the use of solutions as a ruminator for atony of the proventriculus in ruminants; they normalize the motility of the proventriculus, so they can be prescribed for convulsive contractions of the rumen and vomiting that accompany some feed poisoning.
Hypertonic solutions of sodium chloride are recommended for the following acute and chronic diseases and pathological conditions of animals: atony of the proventriculus, tympany and rumen paresis; poisoning with poisonous plants (white hellebore, buttercups, horsetails, solanine), brewing waste (stillage, spent grain), some pesticides (zinc phosphide, organophosphorus compounds, arsenic preparations, fluorides); medicinal toxicoses (poisoning with nitrofurans); acidosis in pregnant cows; gastrointestinal diseases of young calves (toxic dyspepsia, colibacillosis), accompanied by diarrhea and dehydration.
Hypertonic solutions of sodium chloride are injected intravenously: in large animals and sheep into the jugular vein from a Janet syringe without a piston or a 150-250 ml graduated funnel (solution temperature 35-36 ° C).
For diseases of the proventriculus, adult ruminants are administered a 20% solution of sodium, chloride in a dose of 0.07-0.1 g of dry matter per 1 kg of live weight (optimal dose - 0.08-0.09 g, minimum - 0.07, maximum - 0.1 g); for young cattle (up to 1.5-2 years) - 10% solution at a dose of 0.05-0.06 g of dry matter per 1 kg of weight. For acute atony of the proventriculus, a hypertonic sodium chloride solution is prescribed once; for chronic atony, a course of treatment is carried out, consisting of three to four intravenous infusions of the solution at intervals of 48 hours.
Intravenous administration of hypertonic solutions of sodium chloride in traumatic reticulitis can be used for differential diagnosis with primary atony, since in this case there is only a temporary (within 12-24 hours) increase in rumen contractions, the appearance of appetite and chewing gum, but then the condition becomes the same.
In case of poisoning of ruminants, 10-20% solutions of sodium chloride are injected in the same doses as for diseases of the proventriculus; for horses - 10% solution at a dose of 0.05 g of dry matter per 1 kg of live weight. In case of acute poisoning, the solution is administered twice with an interval of 24 hours, depending on the condition of the animal; for chronic ones, a course of treatment is carried out, consisting of three to four infusions with an interval of 48 hours. During treatment, animals are provided with free access to water.
In order to normalize the acid-base balance during acidosis in pregnant cows (decrease in acid capacity below 300 mg%) and to prevent dyspepsia in newborn calves, 1-1.5 months before calving, four to five intravenous infusions of 20% sodium chloride solution are administered at a dose of 0. 09-0.1 g of dry matter per 1 kg of live weight at intervals of 7-10 days.
For toxic dyspepsia and colibacillosis, calves accompanied by dehydration are administered intravenously on the first day of treatment with a 5% sodium chloride solution at a dose of 0.4 g of dry matter per 1 kg of weight. After intravenous administration of a hypertonic solution, the calves are given warm, slightly salted water (5-7 g of sodium chloride per 1 liter of water) from a teat in an amount of 2-3 liters. On the first day of treatment, colostrum is completely replaced with hypotonic (0.5-0.7%) or saline solution, drinking it ad libitum during feeding hours; on the second day of treatment, calves are given colostrum diluted with saline (2/3, 1/2 and 1/3), and only on the third day sick calves are transferred to whole colostrum. With a significant decrease in body temperature (below 37°C), calves are injected with a 10% solution in the same dosage (0.4 g per 1 kg of weight).
Intravenous infusion of 5-10% sodium chloride solutions is prescribed to calves once (preferably on the first day of illness); re-introduction of the solution is allowed no earlier than after 24 hours.
Undesirable effects of a 20% sodium chloride solution include irritation when it enters the subcutaneous tissue with the formation of limited infiltrates. In this case, immediately after administering the solution, generously lubricate the injection sites with tincture of iodine.
Hypertonic sodium chloride solutions are contraindicated for traumatic pericarditis in ruminants and table salt poisoning; Subcutaneous and intraperitoneal administration of hypertonic solutions is not allowed.
Pigs, birds, especially chickens have increased sensitivity to table salt.