Sodium chloride-SOLOpharm
When carrying out any infusion, it is necessary to monitor the patient’s condition, clinical and biological indicators, it is especially important to evaluate blood plasma electrolytes.
In children, sodium excretion may slow down due to immature kidney function. Therefore, in such patients, repeated infusions should be carried out only after determining the concentration of sodium in the blood plasma.
Hypersensitivity reactions
There is evidence of the development of hypersensitivity reactions or infusion reactions during the use of the drug, including hypotension, pyrexia, tremor, chills, urticaria, rash and itching. If hypersensitivity reactions or infusion reactions occur, the infusion should be stopped immediately and the necessary therapeutic measures taken as indicated.
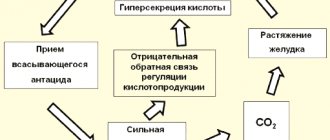
Risk of hypervolemia and/or solute overload and electrolyte imbalances
Depending on the volume and speed of infusion, the following conditions may develop during intravenous administration of the drug:
- hypervolemia and/or solute overload, leading to overhydration and, for example, congestion, including central and peripheral edema;
- clinically significant disturbances in electrolyte and acid-base balance.
Use in patients with renal failure
In patients with renal failure, the drug should be used with extreme caution or not used at all. Use of the drug in such patients may lead to sodium retention.
Use only a clear solution, without visible inclusions and if the packaging is not damaged.
Administer immediately after connecting to the infusion system. The solution should be administered using sterile equipment in compliance with the rules of asepsis and antisepsis. To prevent air from entering the infusion system, it should be filled with solution, releasing any remaining air from the container completely.
As with all parenteral solutions, the compatibility of added substances with the solution must be determined before reconstitution. Drugs known to be incompatible with it should not be used with sodium chloride solution 0.9%. A physician should determine the compatibility of added medicinal substances with a 0.9% sodium chloride solution by checking for possible discoloration and/or the appearance of sediment, insoluble complexes or crystals. Before addition, it is necessary to determine whether the substance being added is soluble and stable in water at the pH level of a 0.9% sodium chloride solution. When adding a drug, it is necessary to determine the isotonicity of the resulting solution before administration.
Before adding drugs to the solution, they must be thoroughly mixed in compliance with aseptic rules. The prepared solution should be administered immediately after preparation, do not store! Do not freeze! Any unused dose should be discarded. Adding other drugs or changing the administration technique may cause fever due to the possible entry of pyrogens into the body. If undesirable reactions develop, you must immediately stop administering the solution.
The Polyflak bottle allows you to dilute dry medications and transfer liquid dosage forms into the bottle using a double-sided cannula for mixing medications.
Freezing during transportation is allowed.
After transportation at subzero temperatures, the drug must be kept at room temperature until completely defrosted.
Freezing the drug, provided the bottle is sealed, is not a contraindication to its use.
Beraxol-solopharm 7.5 mg/ml 100 ml solution for oral administration and inhalation
pharmachologic effect
Expectorant mucolytic agent.
Composition and release form Beraxol-solopharm 7.5 mg/ml 100 ml solution for oral administration and inhalation
Solution - 1 ml:
- Active ingredients: ambroxol hydrochloride - 7.5 mg;
- Excipients: sodium chloride - 6.22 mg, sodium hydrogen phosphate dihydrate - 4.35 mg, anhydrous citric acid - 1.83 mg, benzalkonium chloride - 0.225 mg, water for injection - up to 1 ml;
- Solvent composition (per 1 ml): sodium chloride - 9 mg, water for liquid - up to 1 ml.
Solution for oral administration and inhalation 7.5 mg/ml.
50 or 100 ml of the drug in glass bottles, sealed with a dropper stopper and a lid with first opening control.
1 bottle complete with a measuring cup along with instructions for use in a cardboard pack.
1, 2 or 4 ml of the drug in dropper tube / ampoules A and 1, 2 or 4 ml of solvent in dropper tube / ampoules B made of low-density polyethylene.
10 dropper tubes/ampoules A in a foil film bag and 10 dropper tubes/ampoules B in a foil film bag.
1 or 2 foil film bags with dropper tubes / ampoules A together with 1 or 2 foil film bags with dropper tubes / ampoules B (set) or without them, along with instructions for use in a cardboard pack.
Description of the dosage form
Solution for oral administration and inhalation (bottle or dropper tube / ampoule A) in the form of a clear, colorless or slightly colored liquid; solvent (dropper tube / ampoule B) - transparent, colorless liquid; the prepared solution (drug + solvent) is a clear, colorless or slightly colored liquid.
Directions for use and doses
The mucolytic effect of the drug occurs when taking large amounts of liquid. Therefore, drinking plenty of fluids is recommended during treatment.
Oral use
The drug is taken internally from a dropper tube / ampoule A or a bottle (1 ml = 20 drops from the bottle dropper) after meals, adding to water, tea, milk or fruit juice.
Adults and children over 12 years of age: the first 2-3 days, 4 ml (80 drops) 3 times a day (which corresponds to 90 mg of ambroxol per day), then 4 ml (80 drops) 2 times a day (which corresponds to 60 mg ambroxol per day).
Children from 6 to 12 years old: 2 ml (40 drops) 2-3 times a day (which corresponds to 30 or 45 mg of ambroxol per day).
Children from 2 to 6 years old: 1 ml (20 drops) 3 times a day (which corresponds to 22.5 mg of ambroxol per day).
Children under 2 years of age: 1 ml (20 drops) 2 times a day (which corresponds to 15 mg of ambroxol per day).
For children under 2 years of age, the drug is prescribed only under the supervision of a doctor.
Maximum daily dose when taken orally: for adults – 90 mg, for children 6-12 years old – 45 mg, for children 2-6 years old – 22.5 mg, for children under 2 years old – 15 mg.
Dosage for inhalation
Adults and children over 6 years of age: 1-2 inhalations per day, 2-3 ml of solution (which corresponds to 15-45 mg of ambroxol per day).
Children under 6 years of age: 1-2 inhalations per day of 2 ml of solution (which corresponds to 15-30 mg of ambroxol per day).
Taking the drug for more than 4-5 days is possible only on the recommendation of a doctor.
Pharmacodynamics
Studies have shown that ambroxol increases secretion in the respiratory tract. It enhances the production of pulmonary surfactant and stimulates ciliary activity. These effects lead to increased mucus flow and transport (mucociliary clearance). Increasing mucociliary clearance improves sputum discharge and relieves cough.
In patients with chronic obstructive pulmonary disease, long-term therapy with ambroxol (for at least 2 months) led to a significant reduction in the number of exacerbations. There was a significant decrease in the duration of exacerbations and the number of days of antibiotic therapy.
Pharmacokinetics
All dosage forms of immediate release ambroxol are characterized by rapid and almost complete absorption from the gastrointestinal tract (GIT) with a linear dose dependence in the therapeutic concentration range. The maximum concentration (Cmax) in blood plasma after oral administration is achieved after 1-2.5 hours. The distribution volume is 552 l. In the therapeutic concentration range, binding to plasma proteins is approximately 90%.
The transition of ambroxol from the blood to tissues when administered orally occurs quickly. The highest concentrations of the active component of the drug are observed in the lungs. Approximately 30% of an oral dose is subject to first pass effects through the liver. Studies on human liver microsomes have shown that the CYP3A4 isoenzyme is the predominant isoform responsible for the metabolism of ambroxol to dibromoantranilic acid.
The remainder of ambroxol is metabolized in the liver, mainly by glucuronidation and by partial breakdown to dibromoantranilic acid (approximately 10% of the administered dose), as well as a small number of additional metabolites. The terminal half-life of ambroxol is 10 hours. The total clearance is within 660 ml/min, renal clearance accounts for approximately 8% of the total clearance. Using the radioactive tracer method, it was calculated that after taking a single dose of the drug over the next 5 days, about 83% of the dose taken is excreted in the urine.
Indications for use Beraxol-solopharm 7.5 mg/ml 100 ml solution for oral administration and inhalation
Acute and chronic diseases of the respiratory tract with the release of viscous sputum: acute and chronic bronchitis, pneumonia, chronic obstructive pulmonary disease, bronchial asthma with difficulty in sputum discharge, bronchiectasis.
Contraindications
Hypersensitivity to ambroxol or other components of the drug, pregnancy (first trimester), breastfeeding.
Carefully
Impaired bronchial motor function and increased sputum production (with fixed cilia syndrome), peptic ulcer of the stomach and duodenum during an exacerbation, pregnancy (II-III trimester).
Patients with impaired renal function or severe liver disease should take ambroxol with extreme caution, observing long intervals between doses of the drug or taking the drug in a lower dose.
Application of Beraxol-solofarm 7.5 mg/ml 100 ml solution for oral administration and inhalation during pregnancy and breastfeeding
The drug is contraindicated for use during the first trimester of pregnancy. If it is necessary to use ambroxol in the 2nd-3rd trimesters of pregnancy, the potential benefit to the mother and the possible risk to the fetus should be assessed.
During breastfeeding, the use of the drug is contraindicated, since it is excreted in breast milk.
special instructions
Beraxol-SOLOpharm should not be taken simultaneously with antitussive drugs that may inhibit the cough reflex.
Beraxol-SOLOpharm should be used with caution in patients with a weakened cough reflex or impaired mucociliary transport due to the possibility of sputum accumulation.
Patients taking ambroxol should not be advised to perform breathing exercises; in patients with severe disease, aspiration of liquefied sputum should be performed.
In patients with bronchial asthma, ambroxol may increase cough.
You should not take ambroxol immediately before bed.
In patients with severe skin lesions - Stevens-Johnson syndrome or Lyell's syndrome - fever, body pain, rhinitis, cough and sore throat may appear in the early phase. During symptomatic treatment, it is possible to erroneously prescribe mucolytic drugs such as ambroxol. There are isolated reports of the identification of Stevens-Johnson syndrome and Lyell's syndrome, which coincided with the prescription of the drug, but there is no cause-and-effect relationship with the drug.
If the above syndromes develop, it is recommended to stop treatment and immediately seek medical help.
If kidney function is impaired, ambroxol should be used only on the recommendation of a doctor.
Ambroxol solution is not recommended to be mixed with cromoglicic acid and alkaline solutions.
Patients on a hyposodium diet should keep in mind that 1 ml of ambroxol solution contains 10 mg of sodium. The maximum daily dose for adults and children over 12 years of age contains 120 mg of sodium.
Impact on the ability to drive vehicles and machinery
To date, no cases of the drug affecting the ability to drive vehicles and machinery have been identified.
Overdose
Symptoms: heartburn, dyspepsia, diarrhea, nausea, vomiting, pain in the upper abdomen. There are reports of short-term anxiety. In case of severe overdose, a significant decrease in blood pressure is possible.
Treatment: artificial vomiting, gastric lavage in the first 1-2 hours after taking the drug; intake of fat-containing foods, symptomatic therapy.
Side effects Beraxol-solopharm 7.5 mg/ml 100 ml solution for oral administration and inhalation
The frequency of side effects is presented in the following gradation: very often (≥ 1/10); often (≥ 1/100 to
Gastrointestinal disorders: often – nausea, decreased sensitivity in the oral cavity and pharynx; uncommon – dyspepsia, pain in the upper abdomen, vomiting, diarrhea; rarely - heartburn, dryness of the mucous membrane of the mouth and pharynx, constipation.
Disorders of the respiratory system, chest and mediastinal organs: rarely - dry mucous membrane of the respiratory tract, rhinorrhea.
Nervous system disorders: often – dysgeusia (impaired sense of taste).
Immune system disorders: rarely - hypersensitivity reactions, skin rash, urticaria, itching, angioedema; very rarely - anaphylactic reactions, including anaphylactic shock.
Disorders of the skin and subcutaneous tissues: very rarely - Stevens-Johnson syndrome, Lyell's syndrome.
Other: rarely – adynamia, fever.