Pharmacological properties of the drug Betaxolol
betaxolol (( RS )-l-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-[(1-methylethyl)amino]-2-propan-2-ol) is a selective β1-adrenergic receptor blocker. Due to the cardioselective β-adrenergic blocking effect, it causes a decrease in blood pressure, a decrease in heart rate and a decrease in oxygen consumption by the myocardium. These effects are accompanied by weak negative inotropic and dromotropic effects. At recommended doses, betaxolol does not exhibit partial agonist activity (that is, it does not have BCA) and a membrane-stabilizing effect. Betaxolol reduces serum renin and aldosterone levels. Quickly and completely absorbed after oral administration. The effect of the first passage through the liver is insignificant, bioavailability is about 85%. Approximately 50% of betaxolol binds to plasma proteins. The volume of distribution is approximately 6 l/kg. In the body, betaxolol is mainly converted into inactive metabolites and only 10–15% is excreted unchanged in the urine. The half-life is 15–20 hours. When betaxolol solution is instilled into the eye, increased intraocular pressure is reduced by reducing the production of intraocular fluid. Betaxolol has high lipophilicity, which creates high concentrations of the drug in the tissues of the eye. It begins to act within 30 minutes, and the maximum effect is usually achieved 2 hours after topical application. The duration of reduction in intraocular pressure after a single use is 12 hours.
Betaxolol in the treatment of glaucoma
Betaxolol in glaucoma treatment Betaxolol is a selective antagonist of beta-1-adrenergic receptors. This pharmacological property explains its ability to reduce intraocular pressure (IOP). Betoptic and Betoptic S (Alcon) is used in ophthalmology for treatment of glaucoma and ocular hypertension. It has been shown that despite more IOP decrease versus timolol, betaxolol is superior in visual field preservation in glaucoma patients. This effect can not be explained by adrenergic profile of betaxolol. Recent researches have shown that betaxolol improves ocular blood flow in animals and humans. In the same time it is proven to be able to protect ganglion cells from ischemia in different species of animals. Both ocular blood flow improvement and neuroprotective effect are due to the ability of betaxolol to block calcium channels of cell membrane. Such a range of positive effects on the eye makes betaxolol the drug of choice for POAG treatment.
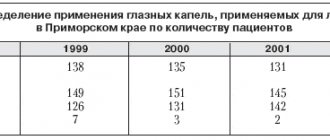
Despite the progress of ophthalmology, the treatment of glaucoma remains one of the most important problems in this field of medicine. Traditionally, drug therapy occupies the main place in the treatment of glaucoma. Thanks to the progress of pharmacology, the arsenal of antiglaucoma drugs has been significantly expanded. However, a large selection of drugs creates certain difficulties for the practicing doctor: it is becoming increasingly difficult to understand the huge flow of scientific and advertising information about new drugs. This review aims to summarize the results of the most important experimental and clinical studies on betaxolol, one of the most effective drugs for the treatment of glaucoma. Betaxolol was first synthesized by Synthelabo for the treatment of cardiovascular diseases. It then began to be used in ophthalmology in the form of eye drops for the treatment of primary open-angle glaucoma and ocular hypertension. The manufacturer of ophthalmic dosage forms of betaxolol under the commercial names “Betoptik” and “Betoptik S” is Alcon (USA). From a pharmaceutical point of view, Betoptik and Betoptik S are two different dosage forms. Betoptik is a 0.5% aqueous solution of betaxolol hydrochloride [18]. Betoptik C is a 0.25% ophthalmic suspension of betaxolol hydrochloride [18]. From the point of view of a practicing physician, these two drugs are interchangeable, since their effectiveness in terms of hypotensive action and other effects is the same [23]. The same, despite different concentrations, effectiveness of Betoptik S (0.25% suspension) compared to Betoptik (0.5% aqueous solution) is due to the use of special polymer technologies in Betoptik S, which increase the bioavailability of the drug [3]. In addition, the use of a special polymer carrier increases the comfort of using the drug and reduces the risk of side effects [3]. According to its pharmacological properties, betaxolol is a selective b1-adrenergic receptor blocker. It does not have internal sympathomimetic activity and membrane-stabilizing (local anesthetic) effect [18]. Like other b-blockers, it reduces intraocular pressure (IOP) by reducing the production of intraocular fluid [22], however, the exact mechanisms of this effect have not yet been fully studied. In addition, there is some improvement in aqueous humor outflow, although in most studies the change in outflow is not statistically significant. Different researchers indicate approximately the same effectiveness of betaxolol in terms of lowering IOP. Regardless of the dosage form (Betoptik or Betoptik S), the drug reduces IOP to the required level in 85–88% of patients [1.19]. Comparing the hypotensive effects of betaxolol and timolol, researchers provide conflicting results. Some believe that it reduces IOP less than timolol [5], others indicate that there is no statistically significant difference in the hypotensive effect of the two drugs [19]. But both agree that betaxolol is superior to timolol in its ability to preserve visual function in patients with glaucoma [6,8,15,19]. For the first time, a more pronounced positive effect of betaxolol on visual fields in patients with glaucoma than timolol was noted in the late 80s - early 90s, when several studies appeared independently of each other confirming this position [5,9,13, 15]. Despite the fact that the design of the experiment and the methods for processing the data obtained in different works differed significantly from each other, the authors obtained very similar results. Thus, the Colignon-Brach study compared the effects of betaxolol and timolol on IOP and retinal sensitivity in patients with primary open-angle glaucoma (POAG) and ocular hypertension for 2 years. IOP was determined using a Goldmann tonometer, retinal sensitivity in the central visual field (30°) was determined using an Octopus automated perimeter. It was shown that despite the weaker hypotensive effect of betaxolol compared to timolol, after 12 months of therapy, the average sensitivity of the retina in patients receiving it statistically significantly increased, while in patients receiving timolol, it decreased. Extension of the study to four years showed that this trend is fully preserved: the average sensitivity of the retina in patients receiving betaxolol is statistically significantly higher than in those receiving timolol [6]. The Colignon-Brach study is in good agreement with the results of another four-year study [19], published in 1996. This study also compared the effects of betaxolol and timolol on IOP and visual fields in patients with POAG. The authors indicate that the hypotensive effect of betaxolol is slightly less than that of timolol, but this difference is not statistically significant. On the contrary, there was a statistically significant difference in the effect of the two drugs on the average retinal sensitivity (ARS) throughout all 4 years of observation. THR in the group of patients receiving betaxolol increased at the very beginning of observation and remained above the initial level until the very end of the study. At the same time, in the group of patients receiving timolol, the heart rate decreased after 18 months of observation and remained below the initial level until the end of the study. It is interesting to note that in the timolol group, three patients underwent trabeculectomy due to significant narrowing of visual fields due to IOP compensation. Studies by Tasindi, Colignon-Brach, Flammer, Kaiser, and others suggest that despite its weaker hypotensive effect, betaxolol has some additional properties that allow it to more effectively preserve visual function than timolol. However, none of the studies reviewed provides an answer to the question of what these properties are. In this regard, the work of Turacli et al. is of interest. [20]. The authors compared the hypotensive effect of betaxolol, its effect on visual fields, blood flow in a. ophthalmica, a. retinalis centralis, aa. ciliares posteriores, measured by Doppler ultrasound, plasma fibrinogen level and erythrocyte sedimentation rate. The study found that, along with improving the results of computer perimetry, under the influence of betaxolol, vascular resistance to blood flow in the a.ophthalmica decreases. These changes turned out to be statistically significant. In addition, vascular resistance decreased in the central retinal artery and posterior ciliary arteries (not statistically significant). The authors conclude that the positive effect on visual fields may be due to the vascular effects of betaxolol. A fairly large amount of literature data on the vascular effects of betaxolol has been accumulated. This review will consider only the most important works. The effect of betaxolol on ocular blood flow and various ocular vessels has been studied by various methods: by measuring pulse ocular blood flow [4], using Doppler ultrasound [7,10,20], in in vitro experiments [12,24]. Summarizing the results of these studies, the following conclusions can be drawn. Firstly, betaxolol increases the speed of linear blood flow in the vessels of the retina and optic nerve head (ONH) [4,7,10,20]. Secondly, betaxolol dilates small arteries and arterioles of the retina and optic disc of animals and humans [7,12,24]. Taken together, this leads to improved blood flow in the retina and optic nerve, which is very important, since microcirculation is usually impaired in patients with glaucoma. The vasodilating effect of betaxolol cannot be explained if we consider this substance only as a b-blocker. Moreover, traditional b-blockers cause vasospasm due to their non-selective effect on vascular b2 receptors. This situation is well known in internal medicine, and for non-selective b-blockers prescribed orally, vasospasm is a known side effect and a contraindication for use. The same data were obtained in ophthalmology. Thus, when instilled into the eye, the non-selective b1,2 adrenergic blocker timolol causes arteriolar spasm in in vivo experiments [21]. This contradiction is easily resolved by data from a number of studies showing that betaxolol also has the properties of a calcium channel blocker. As you know, calcium is necessary for muscle contraction, including the smooth muscles of the vascular wall. It is deposited in the sarcoplasmic reticulum and enters the cell from the intercellular space, in which its concentration is much higher. By opening or blocking the channels through which calcium enters the cell, it is possible to influence the process of muscle contraction. Therefore, drugs in this group, such as nifedipine, have long been used in cardiology to lower blood pressure. The ability of betaxolol to exhibit the properties of calcium channel blockers in ophthalmology has been demonstrated on the vessels of the microvasculature of the eyes in rats [2], pigs [11], cattle [12] and humans [24]. The drug prevents calcium from entering the cell and thereby disrupts the process of muscle contraction. This, in turn, leads to vasodilation and improved microcirculation in the tissues of the eye. It is important to note that betaxolol exerts this effect in the same concentrations in which it is found in the fundus tissues when instilled in therapeutic doses [24]. The ability of betaxolol to block the entry of calcium into the cell determines another property of this drug - its neuroprotective effect. Neuroprotection refers to the ability of a substance to protect neurons from the effects of damaging factors. Conventionally, it can be divided into direct and indirect (mediated) [14]. Indirect neuroprotection can include any effects that improve the living conditions of nerve cells: reducing IOP, improving blood flow, etc. Direct neuroprotection, that is, the effect of a substance directly on a neuron, is of great interest. Thus, immunohistological experiments have shown that betaxolol prevents the death of ganglion cells caused by ischemia in rats, rabbits and primates [16]. This effect is due to the effect of betaxolol on L-type calcium channels and is associated with the metabolism of excitatory amino acids, primarily glutamate. [17]. Thus, summarizing the literature data, we can draw the following conclusions. Along with the hypotensive effect, betaxolol (Betoptik and Betoptik S) has calcium channel blocker properties. This leads to improved microcirculation of the retina and optic nerve head. In addition to this, the ability to block the entry of calcium into the cell determines the neuroprotective effect of betaxolol, which manifests itself in increasing the resistance of ganglion cells to ischemia. All of the above explains the high effectiveness of betaxolol in preserving visual fields in patients with glaucoma (Fig. 1) and allows us to recommend betaxolol (Betoptik and Betoptik S) for widespread use in patients with primary open-angle glaucoma, as a means of choice among other b-blockers.
Literature 1. Erichev V.P. et al. Betoptik S – effectiveness and safety. Materials of the All-Russian scientific and practical conference “Glaucoma at the turn of the millennium: results and prospects.” M.1999 2. Bessho H. et al. Vascular effects of betaxolol, a cardioselective betaadrenoreceptor agonist in isolated rat arteries. Jpn. J. Pharmacol. 1991; 55 : 351–358 3. Betoptic S product monograph, Alcon 1992. 4. Boles Carenini A. et al. Differences in the long-term effect of timolol and betaxolol on the pulsatile ocular blood flow. Surv. Ophthalmol. 1994; 38(suppl):S118–S124 5. Colignon–Brach J. Long-term effect of ophthalmic beta-adrenoreceptor agonists on intraocular pressure and retinal sensitivity in primary open-angle glaucoma. Current Eye Research, vol.11,n1, 1–3, 1992 6. Colignon-Brach J. Long-term effect of topical beta-blockers on intraocular pressure and visual field sensitivity in ocular hypertension and chronic open-angle glaucoma. Surv. Ophthalmol. 1994; 38 (suppl): S149–155 7. Colignon N et al. Effect of topical beta-blockers on human retinal vessels diameter and ocular blood flow. Proceedings of the XI th Congress of European Society of Ophthalmology, Budapest 1997 8. Drance S. Introductory comments on potential differences between beta-blockers in the treatment of open-angle glaucoma. Survey of Ophthalmology, vol 43, suppl 1, June 1999.). 9. Flammer J. et al. A long–term visual field follow up in betaxolol– and timolol–treated patients. In: Perimetry update 1992/1993: Proceedings of the Xth International Perimetric Society Meeting. NY. Kugler Publications, 1993 10. Harris A. et al. Retrobulbar arterial hemodynamic effects of betaxolol and timolol in normal–tension glaucoma. Am. J. Ophthalmol. 1995; 120: 168–175 11. Hester R. et al. The direct vascular releasing action of betaxolol, carteolol ant timolol in porcine long posterior ciliary artery. Surv. Ophthalmol. 1994; 38: S 125–133 12. Hoste A. et al. The relaxant action of betaxolol and propranolol on isolated bovine retinal microarteries. In: Update to glaucoma, ocular blood flow and drug treatment. Cugler Publication NY 1995 13. Kaiser H. et al. Thirty month visual field follow up of glaucoma patients treated with beta blockers. J Glaucoma, 1992; 1: 153–155 14. Levin L. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv. Ophthalmol. 1999, Vol. 43 suppl.1 15. Messmer C, et al. Influence of betaxolol and timolol on the visual field of patients with glaucoma. Am. J. Ophthalmol. 112: 678–681, dec. 1991 16. Osborne N. Neuroprotection and ischemia. In: Proceeding of International glaucoma meeting "Clinical studies in glaucoma on ocular blood flow, neuroprotection and visual field". Istanbul March 25 2000. 17. Osborne N. et al. Topically applied betaxolol attenuates NMDA–induced toxicity to ganglion cells and the effects of ischaemia to the retina. Exp. Eye Res. 1999, 69, 333–342. 18. Physicians' desk reference, 26 edition, Medical Economics. 1998 19. Tasindi E, Talu H., Differential effect of betaxolol and timolol on the progression of glaucomatous visual field loss. A four-year prospective study. Vascular Risk Factors and Neuroprotection in Glaucoma – Update 1996 Cugler publication. NY. 1997 20. Turacli M. et al. The effects of betaxolol on ocular blood flow and visual fields in patients with normotension glaucoma. Eur. J. Ophthalmol. Vol.8 no2, 1998 pp.62–66 21. Van Buskirk E. et al. Ciliary vasoconstriction after topical adrenergic drugs. Am.J.Ophthalmol. 1990; 109:511–517 22. Wax MB, Molinoff PB Distribution and properties of beta-adrenergic receptors in the human iris/ciliary body. Invest. Ophthalmol. 25 (supp.) 305, 1984 23. Weinreb R. et al. A double–masked three–month comparison between 0.25% suspension and 0.5% solution. Am. J. Ophthalmol. 1990; 110: 189–192. 24. Yu D. et al. Effect of betaxolol and Nimodipine on human and pig retimal arterioles. Experiment. Eye Research vol 67 #1 pp.73–81
Use of the drug Betaxolol
Usually prescribed at a dose of 20 mg 1 time per day, however, in some patients, betaxolol is effective at a daily dose of 10 mg. For angina pectoris, the daily dose, if necessary, can be increased to 40 mg. In patients with renal failure with creatinine clearance ≥20 ml/min, no dose adjustment is required. With a creatinine clearance of 20 ml/min and in patients undergoing dialysis, the recommended initial dose is 5 mg/day (regardless of the frequency of dialysis procedures). If the expected effect is not achieved, the dose can be increased by 5 mg/day every 2 weeks to a maximum dose of 20 mg/day. In patients with insufficient liver function, there is no need for dose adjustment, however, clinical observation is advisable at the beginning of therapy. It is recommended to reduce the initial dose to 5 mg in elderly patients, especially those prone to bradycardia. In ophthalmology, adults (including elderly patients) are prescribed 1–2 drops of 0.25% solution into the affected eye(s) 2 times a day. In some patients, it may take several weeks for the antihypertensive effect to stabilize.
Side effects of the drug Betaxolol
Asthenia, cold extremities, bradycardia (sometimes severe), abdominal pain, nausea, vomiting, impotence, dizziness, headache, insomnia, rarely - slowing of AV conduction or progression of existing AV blockade, heart failure, arterial hypotension, bronchospasm, hypoglycemia , Raynaud's syndrome, increased intermittent claudication, skin reactions, including psoriasis-like rashes or exacerbation of psoriasis, depression. In rare cases, the appearance of antinuclear antibodies is observed, which in exceptional cases is accompanied by clinical manifestations such as systemic lupus erythematosus, which disappear after discontinuation of betaxolol. Local reactions when using eye drops: immediately after instillation, short-term discomfort in the eyes is possible. Sometimes - punctate keratitis, photophobia, lacrimation, itching, dry eyes, eye pain, decreased visual acuity, allergic reactions, decreased sensitivity of the cornea, edema and anisocoria.
Special instructions for the use of Betaxolol
Treatment should not be stopped abruptly. Abrupt withdrawal of betaxolol in patients with coronary artery disease may result in severe cardiac arrhythmias, myocardial infarction, or sudden death. The dose of betaxolol should be reduced gradually over 1–2 weeks while starting therapy with another drug. β1-adrenergic blockers for COPD can be prescribed only to patients with moderate disease at a low initial dose. Before starting treatment, it is recommended to assess respiratory function. If bronchospasm develops during treatment, β2-adrenergic agonists are prescribed. In patients with compensated heart failure, betaxolol is prescribed in low initial doses. In the future, if necessary, they are gradually increased under strict medical supervision. The dose of betaxolol should be reduced if the resting heart rate is below 50–55 beats/min. Given the negative dromotropic effect of β-adrenergic receptor blockers, betaxolol is used with caution in patients with first-degree AV block. The use of β-adrenergic receptor blockers can lead to a deterioration in the condition of patients with peripheral circulatory disorders (Raynaud's disease or syndrome, arteritis, chronic occlusive diseases of the arteries of the lower extremities). When β-adrenergic blockers are used in combination with α-adrenergic blockers for the treatment of symptomatic hypertension (arterial hypertension) in pheochromocytoma, careful monitoring of blood pressure is required. In patients with diabetes mellitus, betaxolol can mask the symptoms of hypoglycemia (tachycardia, palpitations, sweating). Prescribed with caution to patients with psoriasis, as there are reports of exacerbation of the disease during treatment with β-adrenergic receptor blockers. In patients with a predisposition to severe allergic reactions, especially those caused by the use of iodinated contrast agents or floctafenine, or when undergoing specific desensitization, therapy with beta-adrenergic blockers may lead to the development of severe anaphylactic reactions and a decrease in the effectiveness of epinephrine in normal doses. During anesthesia, β-adrenergic receptor blockers suppress reflex tachycardia and increase the risk of developing arterial hypotension. The anesthesiologist should be informed that the patient is taking a β-adrenergic blocker. If anesthesia is necessary, it is recommended to discontinue the use of betaxolol 48 hours before surgery (this time is sufficient to restore the sensitivity of β-adrenergic receptors to catecholamines). Therapy with β-adrenergic blockers should not be interrupted in patients with coronary insufficiency; it is advisable to continue treatment until surgery, taking into account the risk associated with the development of withdrawal syndrome. In the case of urgent operations, the patient is prescribed premedication with atropine to prevent stimulation of the vagus nerve, and its administration is repeated if necessary. For anesthesia, it is necessary to use agents with minimal cardiodepressive effect. The use of beta-adrenergic blockers reduces intraocular pressure and may affect test results for glaucoma. Patients receiving systemic and local treatment with beta-blockers should be closely monitored due to the possibility of additive effects. β-adrenergic receptor blockers mask the cardiac symptoms of thyrotoxicosis. Betaxolol may give a positive reaction in doping control tests. Betaxolol is not recommended for use during pregnancy. Newborns whose mothers took β-adrenergic blockers may experience bradycardia, respiratory distress syndrome and hypoglycemia, so careful monitoring of newborns in a specialized department is recommended (monitoring heart rate and serum glucose levels during the first 3–5 days of life). β-adrenergic blockers pass into breast milk, so it is recommended to stop breastfeeding during their use.
Betaxolol-SOLOfarm (Betaxolol-SOLOfarm)
Diabetes
Beta-blockers should be prescribed with caution to patients with a tendency to spontaneous hypoglycemia and patients with labile diabetes mellitus, since these drugs may mask the signs and symptoms of acute hypoglycemia.
Thyrotoxicosis
Beta blockers may mask some symptoms of hyperthyroidism (eg, tachycardia). In patients with suspected thyrotoxicosis, beta-blockers should not be abruptly discontinued, as this may cause an increase in symptoms.
Myasthenia gravis
Beta blockers may cause symptoms and signs similar to those of myasthenia gravis (eg, diplopia, ptosis, general weakness).
Surgery
The anesthesiologist must be informed that the patient is taking betaxolol. Before a planned operation, beta-blockers should be gradually (not all at once!) withdrawn 48 hours before general anesthesia, because during general anesthesia, they can reduce the sensitivity of the myocardium to sympathetic stimulation necessary for cardiac function (for example, they can block the action of adrenaline).
Pulmonology
There have been reports of respiratory reactions, including death due to bronchospasm, in patients with bronchial asthma when using some beta-blockers in ophthalmology.
Betaxolol should be prescribed with caution to patients with moderate to moderate bronchial asthma (including a history of asthma) and patients with mild to moderate chronic obstructive pulmonary disease.
Anaphylactic reactions
When betaxolol is used by patients with atopy or a history of severe anaphylactic reactions to various allergens, there may be a more pronounced reaction to repeated administration of these allergens and unresponsiveness to standard doses of epinephrine in relieving anaphylactic reactions.
Betaxolol should be used with caution in patients with severe peripheral circulatory disorders (i.e., patients with severe Raynaud's disease or Raynaud's syndrome, as well as pheochromocytoma).
When administered locally, beta-blockers can enter the systemic circulation and cause undesirable reactions from the cardiovascular, pulmonary and other systems.
Cases of severe respiratory and cardiovascular disorders, including death from bronchospasm in patients with bronchial asthma and death from heart failure, have been described when using betaxolol.
Heart disorders
In patients with cardiovascular disease (eg, coronary artery disease, Prinzmetal's angina, heart failure) and arterial hypotension, beta-blocker therapy should be critically evaluated with consideration of the possibility of treatment with drugs from other groups. Careful monitoring of the development of signs of exacerbation of the disease and adverse reactions in patients suffering from cardiovascular diseases is necessary.
Corneal diseases
Beta blockers may cause dry eyes. In patients with corneal diseases, the drug should be used with caution.
The main pathogenetic aspect of the treatment of angle-closure glaucoma is the need to open the anterior chamber angle, which is achieved by narrowing the pupil with the help of miotics. Betaxolol does not affect pupil diameter, therefore, in case of angle-closure glaucoma, the drug should be used only in combination with miotics.
Choroidal detachment
Cases of choroidal detachment have been described when using drugs that reduce the production of intraocular fluid (for example, timolol, acetazolamide) after fistulizing antiglaucomatous operations.
This product contains the preservative benzalkonium chloride, which may cause eye irritation and discoloration of soft contact lenses. Direct contact of the drug with soft contact lenses should be avoided. Patients using contact lenses should remove the lenses before using the drug and put them back no earlier than 15 minutes after instillation. With long-term use of the drug by patients with dry eye syndrome and corneal diseases, the development of punctate/ulcerative toxic keratopathy is possible. Patients with corneal diseases should be closely monitored during treatment with the drug.